Available Technologies
# of Displayed Technologies: 30 / 53
Categories
Software for Data-Driven Detection of Space-Time Profiles of Events Detected on the EEG Manifold
TS-004706 — Based on delayed co-detection, this software detects space-time profiles through a data-driven approach.
- College:
- Inventors: Malerba, Paola; Snedden, Ali
- Licensing Officer: Murrah, Kyle
Inhibition of Purinergic Signaling to Promote Neotissue Formation and Prevent Pathological Remodeling in Tissue Engineered Vascular Conduits, Patches, and Valves.
TS-004524 — In engineered cardiovascular tissues, inhibiting purinergic signaling will promote high-quality neo tissue formation and prevent pathological remodeling. Inhibiting the P2Y12 receptor sustains a multitude of other necessary functions but prevents a detrimental excess.
To promote high quality neo tissue formation and inhibit pathological remodeling within engineered cardiovascular tissues, purinergic signaling must be inhibited. The P2Y12 receptor inhibitor called prasugrel, or Effient®, reduces the rate of thrombotic cardiovascular (CV) events (including sten…
- College:
- Inventors: Breuer, Christopher; Reinhardt, James; Turner, Mackenzie
- Licensing Officer: Murrah, Kyle
Lip Guard/Retractor Device
TS-004523 — The LabraGuard lip retractor device intends to prevent injury during oral surgery. The unique design allows independent functioning of each segment for improved patient outcomes.
The LabraGuard is designed to prevent oral injuries by retracting the lips during procedures such as a tonsillectomy, transoral surgery or adenoidectomy. Two narrow connecting pieces fit into each of the three parts of the guard, which allows each segment to function independently. This prevents any…
- College:
- Inventors: Jatana, Kris; Elmaraghy, Charles
- Licensing Officer: Murrah, Kyle
Software Assisted Morbidity & Mortality (M&M) Conferencing
TS-002702 — Patient safety is always a priority for any healthcare setting. Researchers at Nationwide Children’s Hospital developed the Software Assisted Morbidity and Mortality Conferencing platform that was adopted from a previous paper-managed process. The web-application improves patient safety throug…
- College:
- Inventors: Hampl, Josh; Besner, Gail; Kenney, Brian; Murnane, Jami
- Licensing Officer: Murrah, Kyle
ACTIVE-mini 2.0 (BabySure)
TS-002384 — Movement disorders in the United States are often diagnosed after the infant stage. However, early detection and intervention can result in better outcomes. Physical therapists and researchers at Nationwide Children’s Hospital developed the BabySure which detects abnormal movements early on. The device uses color tracking and a depth camera system to track movements in a 2-minute video, which is then compiled to generate a Motor Function Score to compare to typical age-matched peers for early identification or to monitor movement changes in children with known movement impairments.
- College:
- Inventors: Lowes, Linda; Alfano, Lindsay
- Licensing Officer: Murrah, Kyle
Understanding the Clinical Care Practices in LGMD: Patient-Facing Survey
TS-002380 — Limb-girdle muscular dystrophy (LGMD) is a group of disorders that causes weakness in the arms and legs with proximal muscles being the most affected. Currently, there is no standard of care established for LGMD. Physical therapist and researcher, Lindsay Alfano, PT, DPT, PCS, at Nationwide Children’s Hospital’s Center for Gene Therapy created a survey to further understand current diagnosis and patient care practices for LGMD. The survey is designed for families, patients, caregivers, and individuals to gauge the patient’s background and LGMD history (first diagnosis, more frequently seen healthcare providers, testing done, use of assistive devices, access to therapy, etc.). By understanding LGMD patient care through the patient’s perspective, the survey moves the healthcare field one step closer to establishing the best practices of care for LGMD.
- College:
- Inventors: Alfano, Lindsay
- Licensing Officer: Murrah, Kyle
Quantifying Skeletal Muscle Perfusion Using Dynamic PET Imaging of Fluorine-18-Labeled Radionuclides
TS-002316 — Musculoskeletal conditions affect 1.71 billion people worldwide. Researchers at Nationwide Children’s Hospitals developed a novel dynamic approach of PET imaging using commercially available fluorine-18-labeled radionuclides to quantify absolute measures of skeletal muscle perfusion.
By utilizing 18F-labled radionuclides instead of cyclotron production of oxygen-15 water, the methodology is widely accessible to any healthcare system possessing a PET camera by lowering the costs and limiting the need of highly trained radiopharamcy teams to produce and administer oxygen-15 water. Additionally, the methodology will quantify skeletal muscle perfusion for a wide range of muscoskeletal applications and quantify abnormalties from muscle astrophy: any vascular or muscoskeletal disease resulting from underlying ischemia to muscle, ischemic vascular disease, spinal muscular disease, Charcot-Marie-Tooth, etc.
- College:
- Inventors: Stacy, Mitchel; Chou, Ting-Heng
- Licensing Officer: Murrah, Kyle
HEK293 - MIB1-FLAG & HEK293 - HA-Ubiquitin
TS-002315 — The standard human embryonic kidney 293 (HEK293) cell line is widely used in cancer research, vaccine production, adenovirus and adeno-associated viral vectors and gene therapy. Researchers at Nationwide Children’s Hospital have discovered a new use for the HEK293 cell line: generating stable HEK293 cells expressing MiB1-FLAG and hemagglutinin (HA)–ubiquitin. MiB1-FLAG plays a vital role in cell migration and ubiquitin marks HA for degradation and affects its activity. As a result, the HEK293 cells establish a proper immune response, wound repair and tissue homeostasis while attacking hemagglutinin?????
- College:
- Inventors: Garg, Vidu; Majumdar, Uddalak
- Licensing Officer: Murrah, Kyle
 Development of AAV gene therapy for eIF2B5 related vanishing white matter disease
Development of AAV gene therapy for eIF2B5 related vanishing white matter disease
TS-002177 — Researchers at Nationwide Children's Hospital are in the process of developing an Adeno-Associated Virus (AAV) gene therapy for the Eukaryotic Initiation Factor 2B complex (EIF2B5) related Vanishing White Matter Disease (VWM), an inherited pediatric leukodystrophy disease resulting from autosomal recessive mutations in the five subunit genes of EIF2B5. VWM deteriorates the central nervous system’s white matter which affects the brain’s communication and function. Common symptoms include spasticity, ataxia, hypotonia, speech issues, dysphagia, vision and hearing impairments along with cognitive deficits.
The research team is evaluating the CSF delivery of AAV serotype 9 that will target astrocytes which are central in VWM pathology in order to constitute potential therapeutic targets. The AAV vectors will provide wildtype copies of EIF2B5 to address the loss of function resultant from mutations.
- College:
- Inventors: Bradbury, Allison; Flanigan, Kevin
- Licensing Officer: Murrah, Kyle
 Methods for Anticipating Antibiotic Sensitivity in Bacteria Released from Biofilm Residence
Methods for Anticipating Antibiotic Sensitivity in Bacteria Released from Biofilm Residence
TS-002176 — In order to effectively treat bacterial infections, a clear understanding of the bacterium’s antibiotic sensitivity is needed. Researchers at Nationwide Children’s Hospital’s Center for Microbial Pathogenesis created a new method to assist in prescribing antibiotics for infections caused by a biofilm to reduce the dosage and the length of antibiotic treatments.
Depending on the bacteria’s physiologic state the antibiotic sensitivity can be highly variable. Originally, bacteria were believed to exist in two physiologic states: planktonic and biofilm. However, the research team based their methods on two additional but transient physiologic states they…
- College:
- Inventors: Bakaletz, Lauren; Goodman, Steven
- Licensing Officer: Murrah, Kyle
 Smart Myometry Project
Smart Myometry Project
TS-002172 — Current myometers on the market provide inconsistent results, reducing their reliability. Lindsay Alfano, PT, DPT, PCS at Nationwide Children’s Hospital proposed the creation of a new system called Smart Myometry to limit variability and to make strength testing more reliable.
The initial prototype of the device used a steel U-shaped frame that was customizable to the patient’s proportions and reduced the physical therapist’s needed force to resist the muscle. During testing, physical therapists were able to monitor signs of compensation and to detect the need…
- College:
- Inventors: Alfano, Lindsay
- Licensing Officer: Murrah, Kyle
 Virtual Realty for Distraction-Based Pain Therapy in Children and Adolescents
Virtual Realty for Distraction-Based Pain Therapy in Children and Adolescents
TS-002171 — Approximately 30% of children and adolescents experience chronic pain. Researcher, Vanessa Olbrecht, MD, at Nationwide Children’s Hospital developed the FOREVR VR, a device that aims to help patients learn how to regulate their nervous system by maintaining their breathing and heart rate variability.
The device records a patient’s heart rate and respiratory rate to send to the virtual reality game. Patients must accomplish targeted physiological parameters to gain points and unlock new levels. By progressing through the game, the patient simultaneously learns to manage their pain.
- College:
- Inventors: Olbrecht, Vanessa
- Licensing Officer: Murrah, Kyle
 3D Printed Tracheal Bioreactor for Partial Decellularization
3D Printed Tracheal Bioreactor for Partial Decellularization
TS-002170 — With the lack of replacement tissue for airway reconstruction, researchers at Nationwide Children’s Hospital developed the 3D Printed Tracheal Bioreactor for Partial Decellularization. Their new process, Partial Decellularization, treats allografts for tracheal replacement. During testing of small animal subjects, they found that allografts supported epithelial regeneration. They then 3D printed the Bioreactor for the translation and adaptation of Partial Decellularization to human-sized grafts. As a result, Partial Decellularization with the bioreactor simultaneously removed immunogenic cell types of the trachea while preserving the immunoprotected cartilage.
This technology is easily accessible to medical centers who are interested. The creation and assembly of the Bioreactor through 3D printing allows it to be easily sealed, assembled and reduces the risks of contamination making it unlike any other bioreactor on the market.
- College:
- Inventors: Chiang, Tendy; Byun, Woo Yul; Liu, Lumei
- Licensing Officer: Murrah, Kyle
 A Novel 3-D Printed Multi-Organ-on-a-Chip
A Novel 3-D Printed Multi-Organ-on-a-Chip
TS-002169 — Existing models of Organ-on-a-Chip cost more to produce and have limited ability to functionally recapitulate human native tissues due to their limited incorporation of cell types. Researchers at Nationwide Children’s Hospital developed an improved model with their microfluidic Organ-on-a-chip fabrication based on 3D printing. This model optimizes and improves formulation of an extracellular matrix (ECM) that mimics the lamina propria (LP) to sustain the attachment and expansion of different cell types meaning this innovative model has cellular complexity that allows it to mimic human organs and replace animal models in various research settings.
- College:
- Inventors: Mihi, Belgacem; Besner, Gail
- Licensing Officer: Murrah, Kyle
 Neuregulin-1 as Protection from Respiratory Viral Infections
Neuregulin-1 as Protection from Respiratory Viral Infections
TS-002168 — Children have a higher chance of morbidity and mortality from respiratory viral infections. Severe respiratory viral infections like Respiratory Syncytial Virus (RSV) and Parainfluenza viruses can lead to the development of asthma in patients. Clinical researchers at Nationwide Children’s Hospital found that neuregulin-1 (Nrg-1) may be an effective and protective treatment for patients diagnosed with severe respiratory viral infections. Their successful models with mice showed that Nrg-1 may prevent post-viral airway disease and reduce mortality if further studied and applied to human patients in the future.
- College:
- Inventors: Grayson, Mitchell; Hussain, Rehan
- Licensing Officer: Murrah, Kyle
 Second Generation Closed Seeding System for the Tissue Engineered Vascular Graft
Second Generation Closed Seeding System for the Tissue Engineered Vascular Graft
TS-001227 — A team of physicians at Nationwide Children's Hospital have developed a process to improve the acceptance of implanted vascular grafts. This Tissue Engineered Vascular Graft (TEVG) is patient-specific. It seeds patient cells onto a biodegradable tubilar scaffold, which is designed to dissolve with hydrolysis so that only the growing vessel remains. This system, the Closed Seeding System, combines patient imaging data, 3D-printing capabilities, and efficient collection and subsequent seeding of patient cells onto the TEVG scaffolding.
- College:
- Inventors: Breuer, Christopher; Hibino, Narutoshi
- Licensing Officer: Murrah, Kyle
 Sweat Technology for Monitoring Cystic Fibrosis Health and Adherence
Sweat Technology for Monitoring Cystic Fibrosis Health and Adherence
TS-001225 — Cystic fibrosis is an inherited disorder that affects cells that produce sweat and mucus, causing significant damage to the digestive system, lungs, and other organs. A team at Nationwide Children's Hospital has developed a non-invasive monitoring system to track and test a patient with this disease. This technology is a skin patch that measures the metabolomics of the patients sweat to evaluate the clinical health of patients afflicted with cystic fibrosis.
- College:
- Inventors: Hayes, Don; Kopp, Benjamin; Woodley, Frederick
- Licensing Officer: Murrah, Kyle
 Preference Cards and Decision Aid to Facilitate Shared Decision Making in Contraceptive Counseling
Preference Cards and Decision Aid to Facilitate Shared Decision Making in Contraceptive Counseling
TS-001224 — Decks of cards have been used to facilitate knowledge for decades. A team of researchers led by Dr. Elise Berlan have developed a series of cards that combine summaries of contraceptive counciling information and patient preferences. This includes key components of contraceptive preferences which can then be used with a care provider to determine the best form of contraceptive for the patient's preference and decreases the stigma associated with discussing topics like contraceptives as adolecents or young adults.
- College:
- Inventors: Berlan, Elise
- Licensing Officer: Murrah, Kyle
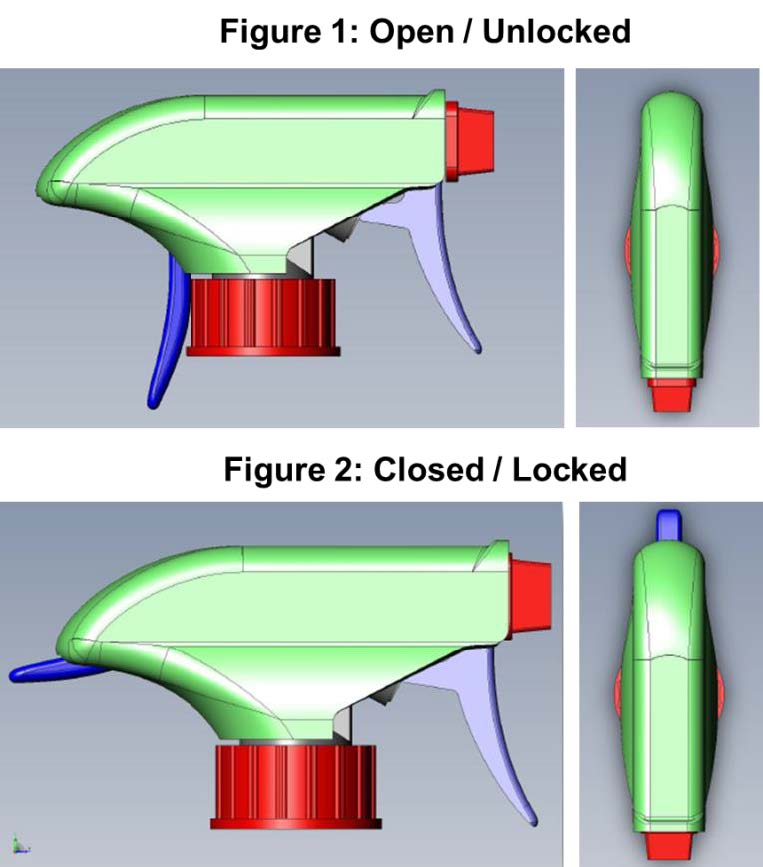 Child-Restraint Spray-Bottle for Household Cleaning Products
Child-Restraint Spray-Bottle for Household Cleaning Products
TS-001037 — When it comes to the safety of our children, innovation never stops. There have been many improvements to the safety of devices and receptacles that can be toxic or life threatening if consumed or exposed to skin. A team of researchers at Nationwide Children’s Hospital have incorporated this desire for security into a common household product: the spray bottle. Often filled with harmful chemicals, spray bottles remain one of the leading causes of chemical exposure injuries in children. The team at NCH has developed a “two-step authentication” spray nozzle that requires the dexterity beyond that of a small child. This dual trigger approach requires a full grip that prevents kids from accessing the contents of the spray bottle, while remaining easily usable by adults and seniors.
Benefits and Applications:
Inventors anticipate that incorporating this product into households will result in a decline in child injury due to accidental activation of spray bottles.
Stage of Development:
- College:
- Inventors: McKenzie, Lara; Nelson, Nicolas; Roberts, Kristin
- Licensing Officer: Murrah, Kyle
 A Virtual Reality Simulation to Aid in Exposure to Therapy for School Avoidance
A Virtual Reality Simulation to Aid in Exposure to Therapy for School Avoidance
TS-001036 — School can be a daunting experience. Constant motion, public speaking and a new environment can increase anxiety in children, sometimes leading to school avoidance. A team at Nationwide Children’s Hospital has developed a solution, where exposure therapy procedures are combined with modern technology can improve the school experience for people of all ages. Using a Virtual Reality (VR) Simulation, clinicians are able to use the multi-user capability to interact with and guide their patient through new environments such as classrooms, hallways and lunchrooms, as well as scenarios known to trigger increased anxiety such as public speaking or asking for help. Biofeedback components help collect data so that the clinician can adapt the experience to the user. Although targeted for school-aged children, this technology can be modified to treat any person with school or public phobia.
- College:
- Inventors: Huang, Yungui; DeForte, Shelly; Luna, John "John"; Mackner, Laura ; Vickery, Elizabeth
- Licensing Officer: Murrah, Kyle
 RyaBhata: A Shiny R Application for Single Cell Transcriptome Data Analysis and Visualization
RyaBhata: A Shiny R Application for Single Cell Transcriptome Data Analysis and Visualization
TS-001035 — Shiny R is an open source platform that allows a framework to develop online applications. With minimal required background in coding principles, a team of researchers at Nationwide Children’s Hospital were able to display and interact with the analysis made of single-cell transcriptome. Shiny R generates visualizations that include UMAP plots and presents features of single-cell RNA and transcriptomic data without extensive training in R programming. Improvements made on this existing technology includes importing data, cell filtration, principle component analysis, clustering, dimensional reduction, merging datasets, and Graphical user interface (GUI)-based generations of gene expression plots. This significantly improves the visualization and analysis of single-cell transcriptome analysis.
- College:
- Inventors: Manivannan, Sathiyanarayanan ; Garg, Vidu
- Licensing Officer: Murrah, Kyle
 Small Molecules that Inhibit and Disperse Salmonella Biofilms in vitro and are Active in Combination with Ciprofloxacin in vivo
Small Molecules that Inhibit and Disperse Salmonella Biofilms in vitro and are Active in Combination with Ciprofloxacin in vivo
TS-001034 — Salmonella is often caused by contact with animals that carry bacteria, contaminated food, or water. It has been observed that children are commonly afflicted by salmonella, and typically treatment includes fluids, medical care, and sometimes pharmaceuticals. A team of researchers at Nationwide Children’s hospital have identified a lead compound that includes a biofilm with anti-salmonella characteristics and acts as an inhibitor. Use of this compound with the antibiotic ciprofloxacin improves the elimination of bacterial infection in at-risk organs such as the liver and spleen.
- College:
- Inventors: Gunn, John; Sandala, Jenna
- Licensing Officer: Murrah, Kyle
 Automated Processing of Venous Intravascular Ultrasound Image
Automated Processing of Venous Intravascular Ultrasound Image
TS-001033 — Intravascular Ultrasound Images (IVUS) is a process that uses micro technology to provide images of blood vessels, their inner walls (endothelium) and the inside of veins. The analysis of these images allows clinicians to analyze luminal and scaffold boundaries, identify the presence of stenosis, and perform computations of various geometric quantities. This process is fully automated and therefore eliminates inconsistencies and inefficiencies that are a direct result of current semi-automated or complex fully automated systems already in place.
- College:
- Inventors: Ulziibayar, Anudari
- Licensing Officer: Murrah, Kyle
 Use of Tamoxifen to Reduce Breast Implant Capsule Formation and Capsular Contracture
Use of Tamoxifen to Reduce Breast Implant Capsule Formation and Capsular Contracture
TS-001029 — A major complication associated with breast implant prostheses is the occurrence of capsular contracture, occurring in 20%-25% of patients. Severe forms of capsular contracture constitute failure of the reconstruction with significant implications for increased cost owing to an increased need for recurrent medical interventions, as well diminished quality of life for patients. Capsular contracture occurs as the result of the patient’s immunologic ‘foreign body’ response to the implant material. The inventors’ vision is to develop a technology whereby the active metabolites of Tamoxifen (endoxifen) are conjugated to implant biomaterial in a manner allowing for localized delivery of endoxifen. They anticipate that local delivery of endoxifen will successfully reduce capsule formation around implant material by reducing the immunologic foreign body response. This is a technology that could be licensed to implant manufacturers (breast, implantable cardiac devices, etc.)
Technology Overview:
Breast augmentation and reconstruction is a common practice, especially in those afflicted by breast cancer. One of the most common issues that comes with this process is the formation of capsular contracture. This is a direct result of the patient’s immunologic ‘foreign body’ response to the implant material, which can impact the need for significant medical interventions and diminished quality of life. The current pharmacologic treatment for breast cancer is the chemical compound known as Tamoxifen, which acts as a chemo-preventative medication for hormone sensitive breast cancers. A team of researchers at Nationwide Children’s Hospital and Ohio State University aims to localize the delivery of tamoxifen to significantly reduce the immunologic foreign body response around implant material for use in both cancer-based breast reconstruction and cosmetic procedures
Benefits:
No pharmacotherapeutics currently exist to address capsular contracture and no biomaterial advances have been made to specifically reduce the foreign body response to breast implants
Stage of Development:
Mouse studies are currently underway using systemic delivery of Tamoxifen for treatment of capsular contracture in breast implants. A manuscript is in preparation.
Future mouse studies will focus on local delivery of endoxifen for treatment of capsular contracture in breast implants; then look at other implant types and different coating types.
Potential Applications / Markets:
According to the report published by Allied Market Research, the medical implant industry estimated $85.38 billion in 2019, and is estimated to generate $147.46 billion by 2027, manifesting a CAGR of 7.2% from 2020 to 2027. According to a report published by Fortune Business Insights, the breast implant market was worth $2.76 billion in 2019 and is projected to reach $3.05 billion by the end of 2027, exhibiting a CAGR of 7.2% during the forecast period, 2020-2027.
Opportunity / Seeking:
-Licensing
IP Status:
Patent application submitted
- College:
- Inventors: Blum, Kevin
- Licensing Officer: Murrah, Kyle
.png) Virtual Reality-Based Pediatric Traumatic Brain Injury Assessment and Rehabilitation Platforms
Virtual Reality-Based Pediatric Traumatic Brain Injury Assessment and Rehabilitation Platforms
TS-000621 — Traumatic brain injury (TBI) is a leading cause of acquired disability in U.S. children and adolescents. Impairment of executive functions post-TBI has broad and profound implications for everyday life of pediatric patients, and the development of effective rehabilitation strategies is of significant clinical importance. Researchers at Nationwide Children’s Hospital have developed virtual reality (VR)-based programs for assessing cognitive function and providing subsequent rehabilitation. This pediatric TBI assessment software provides VR-based cognitive-assessment tasks and an additional training platform that pairs with the Oculus Rift virtual reality viewer. The training program is designed with a series of environmentally-enriched three-dimensional cognitive exercises that aid in rehabilitation of executive core functions among pediatric patients with TBI in a highly controlled, safe, and automated manner.
- College:
- Inventors: Xiang, Henry; Patterson, Jeremy; Shen, Jiabin
- Licensing Officer: Murrah, Kyle
.png) Closed Seeding System for the Tissue Engineered Vascular Graft
Closed Seeding System for the Tissue Engineered Vascular Graft
TS-000620 — Physicians at Nationwide Children’s Hospital have developed a Tissue Engineered Vascular Graft (TEVG) by seeding patient cells onto a biodegradable tubular scaffold. The scaffold degrades by hydrolysis, ultimately leaving only the growing vessel in the patients. The Closed Seeding System enables efficient collection and seeding of patient cells onto the TEVG scaffold, which has been further optimized by using patient imaging data and 3D-printing capabilities to create patient-specific vascular grafts for implantation.
- College:
- Inventors: Breuer, Christopher; Best, Cameron ; Strouse, Robert
- Licensing Officer: Murrah, Kyle
.png) Closed Seeding System for the Tissue Engineered Vascular Graft
Closed Seeding System for the Tissue Engineered Vascular Graft
TS-000619 — Physicians at Nationwide Children’s Hospital have developed a Tissue Engineered Vascular Graft (TEVG) by seeding patient cells onto a biodegradable tubular scaffold. The scaffold degrades by hydrolysis, ultimately leaving only the growing vessel in the patients. The Closed Seeding System enables efficient collection and seeding of patient cells onto the TEVG scaffold, which has been further optimized by using patient imaging data and 3D-printing capabilities to create patient-specific vascular grafts for implantation.
This is an improvement on an existing product. This second-generation system possesses all of the advantages of the previous system including improved safety, efficacy and simplicity. In addition, it has the potential for further improved efficacy and better efficiency.
Benefits:
Using current methods, few hospitals can perform implantations of tissue engineered vascular grafts. TEVG preparation requires advanced collection methods for the cells to be seeded onto the scaffold of the TEVG and must be performed in a sterile environment. The Closed Seeding System enables surgeons to collect patient cells and prepare the TEVG in a standard surgical setting, greatly expanding the availability of this surgery.
Further Details/Stage of Development:
The Closed Seeding System has been tested in an animal model of TEVG implantation.
Potential Applications/Markets:
Initial application of the technology would be used to seed a biodegradable tubular scaffold with autologous bone marrow derived mononuclear cells while simultaneously re-transfusing the residual bone marrow cells (primarily red blood cells) back to the patient. This technology could be used in any application where a specific component of a patient’s blood or bone marrow is removed and processed and the residual cells are returned to the donor.
Opportunity/Seeking:
Development Partner
Commercial Partner
Licensing
IP Status:
Patent Pending
- College:
- Inventors: Breuer, Christopher; Hibino, Narutoshi
- Licensing Officer: Murrah, Kyle
.png) Methods of Treating and Preventing Intestinal Injury Related to Hemorrhagic Shock and Resuscitation
Methods of Treating and Preventing Intestinal Injury Related to Hemorrhagic Shock and Resuscitation
TS-000610 — Hemorrhagic shock and resuscitation (HS/R)-induced injuries often result from trauma or severe blood loss and can quickly progress to organ failure. Researchers at Nationwide Children’s Hospital have developed a novel method for treating subjects at risk for HS/R by administering Heparin Binding-Epidermal Growth Factor (HB-EGF). Administration of HB-EGF protects intestinal epithelial and endothelial cells from HS/R-induced injury in a rat model. This novel method may have broad clinical availability for treating or preventing a range of intestinal injuries in pediatric and adult patients.
- College:
- Inventors: Besner, Gail; El-Assal, Osama
- Licensing Officer: Murrah, Kyle
.png) Neuromuscular GRO worksheet
Neuromuscular GRO worksheet
TS-000596 — Spinal Muscular Atrophy (SMA) is a severe neuromuscular disease and the leading genetic cause of infant mortality. Moreover, existing treatments suffer from notable floor and ceiling effects and also poorly discriminate improved motor performance in patients. To circumvent these challenges, researchers at Nationwide children’s have developed the Neuromuscular Gross Motor Outcome (GRO) worksheet. The GRO worksheet is a gross motor outcome measure designed to assess whole body strength, motor development and function for all levels of ability across the lifespan in those diagnosed with SMA. Hence, the GRO worksheet is the ideal outcome measure tool for SMA or similar conditions to answer the need to quantity gross motor ability across a wide age span.
- College:
- Inventors: Lowes, Linda; Alfano, Lindsay; Iammarino, Megan; Reash (Miller), Natalie
- Licensing Officer: Murrah, Kyle
 Lowes Lab Ambulatory Status Algorithm (LASA)
Lowes Lab Ambulatory Status Algorithm (LASA)
TS-000501 — Research in the field of neuromuscular disease is increasing at an astonishing pace. However, there is no current standardization in the evaluation of the ambulatory status of patients. Researchers at Nationwide Children’s have devised a guide that stratifies patients into ambulatory statuses for data analysis and group assignment. Unlike the traditional binary stratification, this method adds a third stratification which is very important for clinical trial planning and an accurate assessment of the ambulatory status of patients.
- College:
- Inventors: Lowes, Linda; Reash (Miller), Natalie
- Licensing Officer: Murrah, Kyle
