Available Technologies
# of Displayed Technologies: 50 / 159
Categories
CPO-2 (continuous pulse oximeter) and Play on O-2 and C-3PO
TS-003440 — The Continuous Pulse Oximeter (CPO-2) is a conceptual wearable device designed for infants with Bronchopulmonary Dysplasia (BPD) and other patient populations requiring continuous oxygen monitoring. The National Institutes of Health estimates that 10,000-15,000 babies born in the United States develop BPD each year. Inspired by the concept of continuous glucose monitors (CGMs), the CPO-2 aims to offer a wireless, adhesive solution to address the challenges associated with traditional pulse oximeters.
The intellectual property involves creating a device that is capable of reading oxygen saturations (spO2) without the need for a separate machine. Unlike current pulse oximeters that are minimally adhesive and connected to a machine via a cord, the CPO-2 would transmit information directly to a smartphone, eliminating the machine and cord, thereby reducing the risk of strangulation and skin breakdown issues. The adhesive component is expected to last for an extended period, potentially 7-10 days or more, reducing the need for frequent probe replacements.
Potential applications include adoption by home care companies, which could provide the CPO-2 to families taking infants or children home on oxygen. The device could find utility across various age groups, offering a less cumbersome method for continuous oxygen saturation monitoring.
RINCH has filed a provisional application (63/533,946) and the inventor continues to develop and refine this technology.
- College:
- Inventors: Ingram, Mindy
- Licensing Officer: Zalucha, Ellen
Predictive Model of Sudden Cardiac Death in Anomalous Aortic Origin of Coronary Artery (AAOCA)
TS-003439 — The Predictive Model of Sudden Cardiac Death in Anomalous Aortic Origin of Coronary Artery (AAOCA) is a patient-specific computational modeling approach to predict ischemia risk in individuals with anomalous coronaries. Anomalous coronaries are variations or abnormalities in the anatomy of the coronary arteries. These anomalies can differ in presentation and severity, with some increasing the risk of heart problems, such as myocardial ischemia (reduced blood flow to the heart muscle), arrhythmias or sudden cardiac arrest. According to The Cleveland Clinic, up to 1% of the population has an anomalous coronary artery, and most cases are not dangerous.
This predictive model utilizes morphological risk factors derived from advanced imaging data to offer a validated method for risk stratification, determining the need for surgery, selecting the type of surgery, assessing the effectiveness of surgery, and evaluating residual ischemia post-surgery.
Because there are no effective risk stratification approaches for AAOCA individuals, predicting these life-threatening events is challenging. AAOCA is a significant cause of sudden death in children, and current understanding of the mechanisms leading to ischemia and sudden cardiac death (SCD) is limited. According to the Nora Eccles Harrison Cardiovascular Research and Training Institute, "SCD is one of the most significant causes of natural deaths globally. Annually, just over 300,000 US adult deaths are a result of sudden cardiac death. Roughly half of all cardiovascular disease deaths are from SCD." The proposed model addresses these gaps, potentially shifting the AAOCA field from uncertainty to clinical solutions.
RINCH has filed a provisional application (63/502,524) for this technology. The inventors are planning additional refinement of the methodology and a clinical trial to validate the model in a prospective patient population. This technology is jointly owned with Georgia Institute of Technology.
- College:
- Inventors: Krishnamurthy, Rajesh
- Licensing Officer: Zalucha, Ellen
Parent-Implemented Oral Nutrition, Eating and Esophageal Regulation (PIONEER) Products
TS-003438 — The Parent-Implemented Oral Nutrition, Eating and Esophageal Regulation (PIONEER) Products enhance development of oral-pharyngeal-esophageal motility in infants. This technology is a novel approach for training muscles in non-verbal patients, including neonates, infants, and individuals who have lost skills due to stroke, trauma, or developmental neuropathology. Unlike existing methods, these products stimulate muscles involved in esophageal peristalsis and airway protection without electrical stimulation. By engaging and pre-conditioning select muscles and nerves, the approach helps prevent developmental delays, cerebral palsy, and chronic eating and swallowing difficulties.
Approximately half a million infants are admitted to NICUs in the USA annually, thus, the technology’s impact on infant feeding and airway issues could be significant. Potential applications range from diagnostic and therapeutic purposes to rehabilitative therapy for chronic tube-fed infants in Neonatal Intensive Care Units (NICUs) and post-discharge.
RINCH has filed a patent application (PCT/US23/81696) and the inventor is continuing to conduct clinical investigations.
- College:
- Inventors: Jadcherla, Sudarshan
- Licensing Officer: Zalucha, Ellen
VSV-based SARS-CoV-2 Vaccine Candidates and IgY Antibody as Prophylactic and Therapeutic Agents Against SARS-CoV-2 and Variants of Concern
TS-003437 — According to the CDC, roughly 1.1 million COVID-19 cases have been reported in the past year, and 81.4% of the U.S. population have received the updated booster dose. This therapeutic agent utilizes the vesicular stomatitis virus (VSV) vector to protect against SARS-CoV-2 and its variants. This innovative approach resulted in heightened immunogenicity of the vaccines compared to currently available options. Specifically, the utilization of the prefusion Spike protein, stabilized with six prolines (preS-6P), component in the vaccine formulation demonstrates superior efficacy in eliciting a robust immune response. Two distinguishing features of this vaccine platform are the proposed intranasal administration and the live attenuated virus design, both of which induce robust mucosal immunity.
In addition to the vaccine platform, the inventors used the platform to develop specific IgY antibodies to SARS-CoV-2 that might be used as a prophylactic, therapeutic or diagnostic antibody.
This technology holds significant promise in the development of effective vaccines to combat SARS-CoV-2 and its variants long-term. RINCH has filed a provisional application (63/452,870) and the inventors continue to evaluate the efficacy of the vaccine candidates in animal models. This technology is jointly owned with The Ohio State University.
- College:
- Inventors: Peeples, Mark; KC, Mahesh
- Licensing Officer: Zalucha, Ellen
Prevention of Sudden Unexplained Infant Death Cell Phone App Idea Proposal
TS-003427 — The proposed cell phone application, currently in the pre-prototype stage, is designed to address the issue of Sudden Unexplained Infant Deaths (SUIDs). Tailored for parents, especially those with high-risk infants, the app integrates interactive features like safe sleep education, a daily checklist, and a secure photo submission mechanism for monitoring a baby’s safe sleep environment.
There are devices on the market that parents can purchase, but they are not affordable or accessible. The proposed app would be a free alternative to the current expensive devices—with an incentivization feature for consistent household application following birthing center discharge. Beyond this, it serves as a tool to increase knowledge, instill safe sleep practices into caregivers’ daily routines, and directly shape safe behaviors. Aligned with Nationwide Children’s Hospital’s Safe Sleep Initiative taught in the Neonatal Intensive Care Unit (NICU), the app is not only educational, but also interactive, enhancing learning retainability.
The device is marketable on a global scale, catering to every birthing center, NICU, and family worldwide. Emblazoned with the Nationwide Children’s logo, it sends a message that “Everyone Matters,” contributing to the publicization and awareness-building for Nationwide Children’s Hospital. In addition, the app aligns seamlessly with NCH’s inclusion and diversity initiatives by providing incentives, such as discount diapers and baby supplies, to all babies up to one year of age, irrespective of race, ethnicity, or gender identity. This comprehensive approach positions the app as not just a potential life-saving solution but also a valuable asset for companies interested in licensing products related to infant safety and well-being.
- College:
- Inventors: Smathers, Jodi
- Licensing Officer: Mills, Joseph
Child Tracheostomy Task Trainer
TS-003412 — 4,800 pediatric patients undergo tracheostomies annually in the U.S., with multiple caregivers throughout their lives requiring training in the appropriate care. Nationwide Children’s Hospital simulation professionals have developed an innovative Pediatric Tracheostomy Task Trainer model. The manikin offers advanced features for more hands-on training, replicating real-life experiences, including applications such as CPR training.
Existing commercially available task trainers have limitations, and the new model addresses these issues. The portable trainer caters to a broad audience, including healthcare professionals and students with tracheostomy tubes. Key skills associated with the device cover suctioning (both open and closed techniques), stoma care, tracheostomy tie changes, and rescue breathing through tracheostomy/face, depending on insertion availability. Notable improvements include advanced airway management and haptic feedback.
The developed prototype has undergone successful training sessions for hospital staff and caregivers at Nationwide Children’s Hospital. Additionally, a provisional patent application has been filed. We are actively seeking commercial partners to license this technology in the training manikin market, projected to reach $2.60 billion by 2028 with an 11.2% compound annual growth rate.
- College:
- Inventors: Heater, Thomas; Coles, Mary
- Licensing Officer: Mills, Joseph
Internal Hip Distraction
TS-002958 — Hip distraction, or arthrodiastasis, is a surgical option for end-stage diseases of the hip, such as Legg Calve Perthes disease, avascular necrosis, and end-stage osteoarthritis. The procedure improves mobility, decreases pain, and prevents further wear to avoid a total hip arthroplasty. However, the standard of care procedure requires an external device that uses large pins that are kept for 3-4 months which can be distressing for pediatric patients.
NCH inventors have developed a completely internal hip-spanning device that allows for distraction and articulated hip motion. This device will likely reduce the problems associated with an external device such as infection, procedure failure, and psychosocial concerns.
- College:
- Inventors: Kadado, Allen
- Licensing Officer: Zalucha, Ellen
Scoliosis 3D Model for Surgical Simulation
TS-002957 — Available 3D spine models do not optimize properties to simulate real-life bones, ligaments, tendons, viscoelasticity, or resistance that is present in a patient. Spine models are used by both surgeons and researchers for surgical planning, simulation, training, research and medical device development.
By optimizing 3D printing materials, NCH inventors can produce patient-specific spine models that replicate the viscoelastic properties of the spine for a more accurate rendition. In addition to precise surgical planning, another benefit of utilizing viscoelastic accurate models is further medical device innovation to optimize surgical outcomes.
- College:
- Inventors: Kadado, Allen
- Licensing Officer: Zalucha, Ellen
 Targeting GSK3β in NK Cells for Enhanced Antitumor Activity.
Targeting GSK3β in NK Cells for Enhanced Antitumor Activity.
TS-002302 — Acute myeloid leukemia (AML) causes myeloid cells to interfere with the production of healthy white blood cells, red blood cells and platelets; patients will experience fatigue, easy bruising, infections, etc. Due to expansion ex vivo with IL-15, AML patients and donors’ natural killer (NK) cells have an increase in glycogen synthase kinase 3 beta (GSK3β) from the loss of cytotoxicity and defective metabolism.
Researchers at Nationwide Children’s Hospital targeted GSK3β in NK cells to promote antitumor activity by expanding NK cells with feeder cells expressing membrane-bound IL-21 without altering the GSK3β levels. They deleted GSK3β using the cas9/RNP and expanding paired-donor knock out and wild-type NK cells. When assessing transcriptional and functional alterations induced by the loss of GSK3β, GSK3β-KO cells demonstrated changes in gene expressions that suggested possible metabolic reprogramming and exhibited 150% higher spare respiratory capacity, a marker for metabolic fitness. By using mbIL21 expansion in the expansion of NK cells and GSK3β in these cells, the upregulation of GSK and drug inhibitors is prevented.
- College:
- Inventors: Lee, Dean; Naeimi Kararoudi, Meisam; Pereira, Marcelo
- Licensing Officer: Corris, Andrew
 Use of CD38 as the Integration Site for Enhanced Function of Gene-Modified Immune Effector Cells
Use of CD38 as the Integration Site for Enhanced Function of Gene-Modified Immune Effector Cells
TS-002301 — CD38 regulates the metabolism and the immunomodulation of tumor microenvironments, making it an essential component to anti-cancer therapies. Researchers at Nationwide Children’s Hospital developed a novel technology using CD38 as a new insertion site for NK and T cells. They generated CAR-NK and CAR-T cells by integrating the DNA encoding CAR in the CD38 locus to enhance anti-tumor activity and improve metabolic function of NK and T cells. Additionally, this can be used in combination with CD38 monoclonal antibodies without risking fratricide.
- College:
- Inventors: Lee, Dean; Naeimi Kararoudi, Meisam
- Licensing Officer: Corris, Andrew
 Chimeric Antigen Receptor Targeting CD38
Chimeric Antigen Receptor Targeting CD38
TS-002300 — Hematologic cancers like leukemia, lymphoma and myeloma are found in 10% of adult cancer cases and 25% in pediatric cancer cases. Researchers at Nationwide Children’s Hospital’s Center for Childhood Cancer have developed a novel single-chain variable fragment (scFv) that targets and binds to CD38. The scFv can generate CD38 binding proteins, including chimeric antigen receptors (CAR), single-chain antibodies, multi-specific engagers, etc. Additionally, the single-chain variable fragments can be incorporated into polyfunctional proteins and have identical binding properties as CD38 antibodies which are used as anti-cancer therapeutics.
- College:
- Inventors: Lee, Dean; Naeimi Kararoudi, Meisam; Troy, Ella
- Licensing Officer: Corris, Andrew
 Development of AAV gene therapy for eIF2B5 related vanishing white matter disease
Development of AAV gene therapy for eIF2B5 related vanishing white matter disease
TS-002177 — Researchers at Nationwide Children's Hospital are in the process of developing an Adeno-Associated Virus (AAV) gene therapy for the Eukaryotic Initiation Factor 2B complex (EIF2B5) related Vanishing White Matter Disease (VWM), an inherited pediatric leukodystrophy disease resulting from autosomal recessive mutations in the five subunit genes of EIF2B5. VWM deteriorates the central nervous system’s white matter which affects the brain’s communication and function. Common symptoms include spasticity, ataxia, hypotonia, speech issues, dysphagia, vision and hearing impairments along with cognitive deficits.
The research team is evaluating the CSF delivery of AAV serotype 9 that will target astrocytes which are central in VWM pathology in order to constitute potential therapeutic targets. The AAV vectors will provide wildtype copies of EIF2B5 to address the loss of function resultant from mutations.
- College:
- Inventors: Bradbury, Allison; Flanigan, Kevin
- Licensing Officer: Murrah, Kyle
 Methods for Anticipating Antibiotic Sensitivity in Bacteria Released from Biofilm Residence
Methods for Anticipating Antibiotic Sensitivity in Bacteria Released from Biofilm Residence
TS-002176 — In order to effectively treat bacterial infections, a clear understanding of the bacterium’s antibiotic sensitivity is needed. Researchers at Nationwide Children’s Hospital’s Center for Microbial Pathogenesis created a new method to assist in prescribing antibiotics for infections caused by a biofilm to reduce the dosage and the length of antibiotic treatments.
Depending on the bacteria’s physiologic state the antibiotic sensitivity can be highly variable. Originally, bacteria were believed to exist in two physiologic states: planktonic and biofilm. However, the research team based their methods on two additional but transient physiologic states they…
- College:
- Inventors: Bakaletz, Lauren; Goodman, Steven
- Licensing Officer: Murrah, Kyle
 Salvianolic Acid (SAA) Treatment of FSHD
Salvianolic Acid (SAA) Treatment of FSHD
TS-002175 — The third most common type of muscular dystrophy, Facioscapulohumeral Muscular Dystrophy (FSHD), affects over 870,000 individuals worldwide by causing debilitating pain, muscle weakness, fatigue along with many other symptoms in their face, shoulders, upper arms and lower legs. Researchers at Nationwide Children’s Hospital created a treatment using Salvianolic Acid (SAA) as a drug therapy for neuromuscular disorders including FSHD.
SAA, a natural compound found in the Salvia plant, has never been used for treating FSHD or any other neuromuscular disorder before. The compound inhibits protein methyltransferase (PRMT1), protects cells from double-homeobox gene 4 (DUX4) induced death and reduces the addition of methyl groups on t…
- College:
- Inventors: Harper, Scott; Eidahl, Jocelyn; Knox, Renatta; Wallace, Lindsay
- Licensing Officer: Eidahl, Jocelyn
 Gene Therapy for CMT1B
Gene Therapy for CMT1B
TS-002174 — Currently, no cure exists for Charcot-Marie tooth type 1B (CMT1B). Inventors and specialists in Gene Therapy at Nationwide Children’s Hospital invented a methodology along with sequences for using microRNAs (miRNA) to inhibit and replace abnormal expressions of the myelin protein zero (MPZ) gene. Affecting 1 in 30,000 people, CMT1B is caused by more than 200 mutations of the MPZ, the essential protein needed for a healthy and efficient peripheral nervous system. The accumulation of mutant MPZ genes will result in, but not limited to, muscle weakness, atrophy, lost of sensation in the lower legs and feet and sensory loss.
These methods can treat, delay the progress of and prevent diseases caused by the mutations. This gene therapy knocks down MPZ gene expression with nucleic acid encoded artificial microRNAs hybridized to target nucleic acid sequences at the mRNA level and a nucleic acid encoding a codon-optimized MP…
- College:
- Inventors: Rashnonejad, Afrooz; Harper, Scott
- Licensing Officer: Eidahl, Jocelyn
 Smart Myometry Project
Smart Myometry Project
TS-002172 — Current myometers on the market provide inconsistent results, reducing their reliability. Lindsay Alfano, PT, DPT, PCS at Nationwide Children’s Hospital proposed the creation of a new system called Smart Myometry to limit variability and to make strength testing more reliable.
The initial prototype of the device used a steel U-shaped frame that was customizable to the patient’s proportions and reduced the physical therapist’s needed force to resist the muscle. During testing, physical therapists were able to monitor signs of compensation and to detect the need…
- College:
- Inventors: Alfano, Lindsay
- Licensing Officer: Murrah, Kyle
 Virtual Realty for Distraction-Based Pain Therapy in Children and Adolescents
Virtual Realty for Distraction-Based Pain Therapy in Children and Adolescents
TS-002171 — Approximately 30% of children and adolescents experience chronic pain. Researcher, Vanessa Olbrecht, MD, at Nationwide Children’s Hospital developed the FOREVR VR, a device that aims to help patients learn how to regulate their nervous system by maintaining their breathing and heart rate variability.
The device records a patient’s heart rate and respiratory rate to send to the virtual reality game. Patients must accomplish targeted physiological parameters to gain points and unlock new levels. By progressing through the game, the patient simultaneously learns to manage their pain.
- College:
- Inventors: Olbrecht, Vanessa
- Licensing Officer: Murrah, Kyle
 3D Printed Tracheal Bioreactor for Partial Decellularization
3D Printed Tracheal Bioreactor for Partial Decellularization
TS-002170 — With the lack of replacement tissue for airway reconstruction, researchers at Nationwide Children’s Hospital developed the 3D Printed Tracheal Bioreactor for Partial Decellularization. Their new process, Partial Decellularization, treats allografts for tracheal replacement. During testing of small animal subjects, they found that allografts supported epithelial regeneration. They then 3D printed the Bioreactor for the translation and adaptation of Partial Decellularization to human-sized grafts. As a result, Partial Decellularization with the bioreactor simultaneously removed immunogenic cell types of the trachea while preserving the immunoprotected cartilage.
This technology is easily accessible to medical centers who are interested. The creation and assembly of the Bioreactor through 3D printing allows it to be easily sealed, assembled and reduces the risks of contamination making it unlike any other bioreactor on the market.
- College:
- Inventors: Chiang, Tendy; Byun, Woo Yul; Liu, Lumei
- Licensing Officer: Murrah, Kyle
 A Novel 3-D Printed Multi-Organ-on-a-Chip
A Novel 3-D Printed Multi-Organ-on-a-Chip
TS-002169 — Existing models of Organ-on-a-Chip cost more to produce and have limited ability to functionally recapitulate human native tissues due to their limited incorporation of cell types. Researchers at Nationwide Children’s Hospital developed an improved model with their microfluidic Organ-on-a-chip fabrication based on 3D printing. This model optimizes and improves formulation of an extracellular matrix (ECM) that mimics the lamina propria (LP) to sustain the attachment and expansion of different cell types meaning this innovative model has cellular complexity that allows it to mimic human organs and replace animal models in various research settings.
- College:
- Inventors: Mihi, Belgacem; Besner, Gail
- Licensing Officer: Murrah, Kyle
 Neuregulin-1 as Protection from Respiratory Viral Infections
Neuregulin-1 as Protection from Respiratory Viral Infections
TS-002168 — Children have a higher chance of morbidity and mortality from respiratory viral infections. Severe respiratory viral infections like Respiratory Syncytial Virus (RSV) and Parainfluenza viruses can lead to the development of asthma in patients. Clinical researchers at Nationwide Children’s Hospital found that neuregulin-1 (Nrg-1) may be an effective and protective treatment for patients diagnosed with severe respiratory viral infections. Their successful models with mice showed that Nrg-1 may prevent post-viral airway disease and reduce mortality if further studied and applied to human patients in the future.
- College:
- Inventors: Grayson, Mitchell; Hussain, Rehan
- Licensing Officer: Murrah, Kyle
 Manipulating Mammalian Gene Expression with Bacterial Extracellular Vesicles
Manipulating Mammalian Gene Expression with Bacterial Extracellular Vesicles
TS-002167 — Research links increased expression of neuregulin-1 (NRG1) to neurological disorders (schizophrenia, bipolar disorder, autism spectrum disorder, epilepsy, etc.), cancers (breast, prostate, lung, gastric cancers), metabolic syndrome and multiple sclerosis. Researchers at Nationwide Children’s Hospital developed a treatment to manipulate mammalian gene expression with bacterial extracellular vesicles. This treatment based on outer membrane vesicles (OMV) modulates expression of NRG1 by using porphyromonas gingivalis (Pg) tRNA and NRG1 mRNA to reduce NRG1 translation. Pg and closely related pathogens produce OMVs that contain proteins, lipids, nucleic acids and cytosolic compounds. Through metagenomic sequencing, researchers found that Pg OMVs contain tRNA that complements mammalian NRG1 mRNA. They also found that the tRNA can potentially stop the translation of NRG1. This complementation indicates a direct interaction between Pg tRNA and NRG1 mRNA which results in the reduction of NRG1.
- College:
- Inventors: Lauber, Christian; Blalock, Lexie; Mashburn-Warren, Lauren
- Licensing Officer: Corris, Andrew
 Caring Contact Service Automation and Management
Caring Contact Service Automation and Management
TS-002166 — Education and awareness about mental health increased the demand for better digital programs so health care professionals can reach patients outside the clinical setting. Researchers at Nationwide Children’s Hospital created a program that sends multimedia text messages to encourage positive thinking and to reduce chances of readmission to behavioral health hospitals.
Before the Caring Contact Service Automation and Management, clinical coordinators needed to manually send text messages to patients and to track their enrollment in the program. With this program, patients will be enrolled into the program and will receive the confirmative messages with configured …
- College:
- Inventors: Ackerman, John; Huang, Yungui; Oiler, Brannon; Thomas, Glenn
- Licensing Officer: Corris, Andrew
 Hair Care Equity Project
Hair Care Equity Project
TS-002165 — Children have higher self-esteem when their needs are supported, which includes hair care. In 2020, staff members reported their knowledge gaps and lack of confidence and supplies regarding patient hair care. As a result, the Hair Care Equity Project was created at Nationwide Children’s Hospital to combat these challenges and to create a more inclusive environment for patients through education and product research. The Hair Care Equity Committee worked with vendors and dermatologists to carefully select products for trials and final selection. Now, staff members can access and order supplies for patients with all hair types.
Their educational tool includes interactive learning modules, instructional videos and charts detailing styling and hair tools. The modules provide in-depth procedure steps for detangling, washing, conditioning, moistrizing and styling keeping cultural considerations, accessories, oxygen safety, etc…
- College:
- Inventors: Asamoa, Surlina; Clark, Nimrah; Hoy, Jimia; Justice, Lauren; Miller, Robbii; Regis, Kimberly
- Licensing Officer: Corris, Andrew
 A Tailored mHealth Vaping Prevention Intervention for Adolescents with Congenital Heart Defects
A Tailored mHealth Vaping Prevention Intervention for Adolescents with Congenital Heart Defects
TS-002164 — Congenital heart defects (CHD) are the most common birth defects with an occurrence rate of 1 in every 110 births. When transferring from pediatric to adult care, one-third of adolescents are in optimal cardiovascular health. Due to this, health behavior intervention is ideal in adolescence. This is especially crucial when youths report using e-cigarettes or the possibility of using one in the future. Researcher, Kristen Fox, at Nationwide Children’s Hospital created a tailored mHealth Vaping Prevention program for adolescents with CHD to reduce engagement with health risk behaviors. The curriculum is improved with added topics surrounding stress management and disease knowledge with the implementation of gamification and animations to facilitate knowledge and sustain engagement about tobacco product education.
By utilizing the mHealth format, the curriculum will reach and engage adolescents with content that classroom-based programs cannot or failed to in the past. For example, the curriculum includes a module about stress management that will cover goal setting, sleep hygiene, problem solving/ coping and…
- College:
- Inventors: Fox, Kristen
- Licensing Officer: Corris, Andrew
 Pediatric and Rare Disease Clinical Research: Your Reference Manual for Navigating a Complex Research Environment
Pediatric and Rare Disease Clinical Research: Your Reference Manual for Navigating a Complex Research Environment
TS-002163 — Researchers and professionals beginning their careers in pediatrics and/or rare disease clinical research sites may feel overwhelmed in the face of innovation, new territories and lifechanging research. A team of Certified Clinical Research Professionals at Nationwide Children’s Hospital created three additional modules for the Clinical Research Onboarding Program – The Survival Guide and an opportunity to access a Gene Therapy Immersion Program. Through this reference manual, the team hopes to share lessons and provide guidance on working in pediatrics clinical research for all learners.
The three modules focus on pediatrics and rare disease clinical research: Pediatric Clinical Research, Pediatric Rare Disease Clinical Research and Study Management for Pediatric and Rare Disease Clinical Research. Like the previous six modules, the new modules are also in-depth and combat challenge…
- College:
- Inventors: Wentzel, Grace; Baker, Christine; Roush, Kandice
- Licensing Officer: Corris, Andrew
 Gastric Secretion Reinfuser (GSR)
Gastric Secretion Reinfuser (GSR)
TS-002162 — Dr. Jonathon Gisser, a pediatric gastroenterologist at Nationwide Children’s Hospital, researched and created a process called Gastric Secretion Reinfusion (GSR) to help salvage gastric fluids by reinfusing them into the jejunum.
Gastrojejunostomy (GJ) tubes cause increased vomiting and/or gastric drainage in patients due to gastric outlet obstruction. The small intestine needs gastric fluids to prevent dehydration, dysmotility, malabsorption, malnutrition, electrolyte imbalances and increased gastric output because they are rich in electrolytes, enzymes and hormones. Currently, gastric fluids are typically discarded and replaced by nutrient-impoverished, exogenous fluids like intravenous fluids (IV). By implementing the Gastric Secretion Reinfusion technique, patients will experience decreased incidences of electrolyte abnormalities, vomiting and reliance on parenteral access and Total Parenteral Nutrition (TPN). Additionally, they will also experience an increase in tolerance of jejunal feeds and improved nutrition.
- College:
- Inventors: Gisser, Jonathan
- Licensing Officer: Corris, Andrew
 Staffing Analysis Tool
Staffing Analysis Tool
TS-002161 — The Staffing Analysis Tool helps organizations track and plan for staffing in the future. Inventors at Nationwide Children’s Hospital developed this smart spreadsheet for workplaces to effectively see staffing levels easily and automatically through its real time updates. Users only need to input staffing needs and staff information into its built-in functions. Once completed, the Staffing Analysis Tool will comprise the information to create an overall picture of staffing insufficiencies including planned leaves, resignations and other challenges. With the final picture calculated, workplaces can always ensure effective staffing.
- College:
- Inventors: Larouere, Marissa; Michael, Jeanine
- Licensing Officer: Zalucha, Ellen
 IDDSI Implementation Manual and Education
IDDSI Implementation Manual and Education
TS-002160 — Dysphagia affects 300,000 to 700,000 Americans every year. Individuals of any age can suffer with dysphagia which increases the demand for education and information. Providers working with individuals with dysphagia require easy, quick access to education and information regarding implementing the International Dysphagia Diet Standardization Initiative (IDDSI). Clinicians at Nationwide Children’s Hospital created the IDDSI Implementation Manual and Education. The manual provides healthcare providers with specific implementation steps, instructions and details to guide teams to successfully integrate IDDSI in their healthcare organization quickly along with additional supplemental educational materials.
- College:
- Inventors: O'Rourke, Sara; Coleman Casto, Shelley; Patton, Rebecca; Stevens, Melanie
- Licensing Officer: Corris, Andrew
 Therapeutic Recreation (TR) Leisure Engagement
Therapeutic Recreation (TR) Leisure Engagement
TS-002159 — Currently, there is no standard protocol for observing patient readiness for recreational therapy. Inventors at Nationwide Children’s Hospital created the Therapeutic Recreation (TR) Leisure Engagement tool to document and assess the needs of patients with developmental or physical disabilities to promote well-being and recovery. Through this technology, therapeutic recreation specialists will further help patients by measuring their readiness for sessions, emotional presentation and social functions while providing leisure education.
- College:
- Inventors: Lazzara Mould, Valerie; Chadbourne, Mary; Sotak, Heather
- Licensing Officer: Corris, Andrew
 Adeno-Associated Virus Delivery of CLN8 Polynucleotide
Adeno-Associated Virus Delivery of CLN8 Polynucleotide
TS-002095 — About 3 of every 100,000 births are affected by Batten disease, a disorder that prevents the body from removing cellular waste like lipids and proteins. The build up of cellular waste throughout the body can cause seizures, vision loss, delays in thinking, abnormal movements and death. Researchers at Nationwide Children’s Hospital created a new intrathecal gene therapy using Adeno-Associated viral vectors for Batten Disease caused by mutations in the CLN8 gene. They generated an Adeno-Associated viral vector serotype 9 (AAV9) that contains the human CLN8 cDNA driven by a promoter.
Additionally, they designed a new concept of intrathecal dosing which calculates the dosage by the size and growth rate of the nervous system and cerebrospinal fluid. The intrathecal gene therapy method can be used for Batten disease caused by mutations in the CLN8 gene along with other gene therapy…
- College:
- Inventors: Meyer, Kathrin; Kaspar, Brian; Likhite, Shibi
- Licensing Officer: Eidahl, Jocelyn
 Occupational Therapy Batten Assessment
Occupational Therapy Batten Assessment
TS-002008 — Effecting three of every 100,000 births, Batten Disease affects the body’s ability to discard cellular wastes like lipids and proteins leading to build up in cells. The build up can cause seizures, vision loss as well as cognitive and mobility impairments. Occupational therapist, Virginia B. Goddard, developed the Batten Disease assessment to better encapsulate patients’ functional abilities. The assessment measures patients’ daily activities, functional fine motor skills, visual abilities, stereognosis abilities, sensory responses and behavior. These categories are broken down into smaller activities and each activity is scored on a scale of 0 to 4: 0 marking minimal to no impairment and 4 marking severe or complete impairment. At the end of the assessment, occupational therapists can calculate a final score out of 76 to measure impairment severity.
- College:
- Inventors: Goddard, Virginia
- Licensing Officer: Eidahl, Jocelyn
 GRIN2D RNAi Gene Therapy to Treat Epilepsy
GRIN2D RNAi Gene Therapy to Treat Epilepsy
TS-002006 — Overexpression of the GRIN2D gene can cause Developmental and Epileptic Encephalopathy (DEE) in which individuals may experience developmental delays or intellectual disabilities, epilepsy, abnormal muscle tone, movement disorders, autism spectrum disorder and cortical visual impairment. Currently, only supportive care is available. Genetic Researchers at Nationwide Children’s Hospital developed a RNAi gene therapy to treat epilepsy caused by GRIN2D. They propose decreasing the expression of GRIN2D through a gene-level specific reagent which will knock down the mRNA containing the variant postnatally. As a result, reducing the possibility of children developing GRIN2D-related DEE.
- College:
- Inventors: Harper, Scott
- Licensing Officer: Eidahl, Jocelyn
 GEMys: Therapeutic Effect of Genetically Engineered Bone Marrow Derived Myeloid Cells in Central Nervous System Tumors
GEMys: Therapeutic Effect of Genetically Engineered Bone Marrow Derived Myeloid Cells in Central Nervous System Tumors
TS-001983 — A research team at Nationwide Children’s Hospital developed a new therapeutic approach for delaying lower grade glioma malignant progression using adolescents and young adult models of glioma.
The innovative approach uses autologous bone marrow isolated myeloid cells (GEMys) for the stable expression and secretion of interleukin 2 (IL2), a cytokine associated with responding to invading pathogens and the recruitment of cytotoxic anti-cancer immune cells to the tumor microenvironment (TME). These bone marrow derived GEMys can also be used to deliver other cytokines to potentiate the trafficking and activation of cytotoxic T and NK cells to kill tumor cells and reprogram the TME to prevent glioma progression. This novel innate immunotherapy is delivered systemically through intravenous injection and may be less toxic than chemotherapies.
- College:
- Inventors: Rajappa, Prajwal; Canella, Alessandro
- Licensing Officer: Corris, Andrew
 AAV Vectors Containing U7small Nuclear RNA (U7snRNA That Interferes With The CTGexp or Silences DMPK)
AAV Vectors Containing U7small Nuclear RNA (U7snRNA That Interferes With The CTGexp or Silences DMPK)
TS-001229 — Mutations in the Myotonic Dystrophy Protein Kinase gene (DMPK) cause an autosomal dominant inherited disease referred to as Myotonic Dystrophy. Myotonic Dystrophy affects more than 1 in 8,000 people worldwide. Myotonic dystrophy results in progressive muscle weakness, stiffness and wasting. Currently, here are no treatments for this disease. A team of researchers at Nationwide Children’s Hospital have designed short hairpin constructs of AAV-shRNA which can be used to silence DMPK mRNA and therefore potentially treat myotonic dystrophy.
- College:
- Inventors: Wein, Nicolas
- Licensing Officer: Eidahl, Jocelyn
 Second Generation Closed Seeding System for the Tissue Engineered Vascular Graft
Second Generation Closed Seeding System for the Tissue Engineered Vascular Graft
TS-001227 — A team of physicians at Nationwide Children's Hospital have developed a process to improve the acceptance of implanted vascular grafts. This Tissue Engineered Vascular Graft (TEVG) is patient-specific. It seeds patient cells onto a biodegradable tubilar scaffold, which is designed to dissolve with hydrolysis so that only the growing vessel remains. This system, the Closed Seeding System, combines patient imaging data, 3D-printing capabilities, and efficient collection and subsequent seeding of patient cells onto the TEVG scaffolding.
- College:
- Inventors: Breuer, Christopher; Hibino, Narutoshi
- Licensing Officer: Murrah, Kyle
 Sweat Technology for Monitoring Cystic Fibrosis Health and Adherence
Sweat Technology for Monitoring Cystic Fibrosis Health and Adherence
TS-001225 — Cystic fibrosis is an inherited disorder that affects cells that produce sweat and mucus, causing significant damage to the digestive system, lungs, and other organs. A team at Nationwide Children's Hospital has developed a non-invasive monitoring system to track and test a patient with this disease. This technology is a skin patch that measures the metabolomics of the patients sweat to evaluate the clinical health of patients afflicted with cystic fibrosis.
- College:
- Inventors: Hayes, Don; Kopp, Benjamin; Woodley, Frederick
- Licensing Officer: Murrah, Kyle
 Preference Cards and Decision Aid to Facilitate Shared Decision Making in Contraceptive Counseling
Preference Cards and Decision Aid to Facilitate Shared Decision Making in Contraceptive Counseling
TS-001224 — Decks of cards have been used to facilitate knowledge for decades. A team of researchers led by Dr. Elise Berlan have developed a series of cards that combine summaries of contraceptive counciling information and patient preferences. This includes key components of contraceptive preferences which can then be used with a care provider to determine the best form of contraceptive for the patient's preference and decreases the stigma associated with discussing topics like contraceptives as adolecents or young adults.
- College:
- Inventors: Berlan, Elise
- Licensing Officer: Murrah, Kyle
 Machine Learning of Doppler Echocardiographic Coronary Blood Flow
Machine Learning of Doppler Echocardiographic Coronary Blood Flow
TS-001178 — Currently there are few existing methods on coronary flow pattern automation. The DECFA platform fulfills this unmet need by predicting diseased coronary blood flow by integrating previously unutilized data features from (sonographic) Doppler echocardiography measurements, cardiac functional and other physiological data (e.g. heart rate, body weight, etc) using machine learning. The DECFA program is superior to manual intervention as it provides more efficient analysis, more accurately, and can accept raw video files of PW Doppler and Color Doppler B-mode files, applicable on mouse models, potentially applicable to humans. DECFA can analyze raw PW Doppler AND Color Doppler B-Mode AVI video files to calculate overall coronary blood flow and coronary flow reserve, and the separation of each coronary flow pattern into 4 distinct phases representative of the stages in a cardiac cycle.
Benefits:
More efficient analysis over manual intervention, less error than manual intervention, capable of accepting raw video files of PW Doppler and Color Doppler B-mode files, applicable on mouse models, potentially applicable to humans (not yet validated). New features include the analysis of raw PW Doppler AND Color Doppler B-Mode AVI video files to calculate overall coronary blood flow and coronary flow reserve, and the separation of each coronary flow pattern into distinct phases representative of the stages in a cardiac cycle. The machine learning aspect brings state-of-the-art technology to determine whether it may be useful in directly predicting/diagnosing coronary microvascular disease.
Stage of Development:
We are currently in the final stages of completing the data analysis for all of the in vivo coronary and cardiac physiological parameters that will be used to perform the final runs through the machine learning process. We did perform an “interim” analysis using about half of the data, the results of which were promising (inconclusive at this point, but they put the predictive value of coronary flow patterns above 90% for identifying diseased coronary blood flow). This process also uses the whole envelope instead of discrete points of the coronary flow pattern, in addition to the texture-analysis extension. After this process is complete in mice, we plan to obtain human coronary blood flow patterns to determine whether this could be clinically useful beyond research applications.
Potential Applications/Markets:
Our program could be utilized in a research setting for robust, comprehensive, and more efficient analysis of coronary flow patterns in mice measured through Doppler Echocardiography (It solves the problem of large inter/intra observer error and time required for manual analysis). The program could also be used clinically for use in the medical field for the same analysis if adjusted for human use. It could also be used as an add on feature to the VisualSonics Vevo 2100 software for added capabilities in analyzing PW Doppler coronary flow patterns and Color Doppler B-mode files. The parameters that we identify in our program could be potentially useful in clinical diagnostics/machine learning/prediction modeling for better identifying and predicting disease.
Furthermore, we envision that this could be tested and applied to clinical coronary Doppler echocardiograms, with the readout being predictability of coronary microvascular disease based on the machine learning algorithm of coronary flow patterns.
Opportunity/Seeking:
Development Partner
Commercial Partner
Licensing
IP Status:
Know-how based
Copyright
- College:
- Inventors: Trask, Aaron; Bartlett, Christopher; Bossenbroek, Jamie; McCallinhart, Patricia; McDermott, Michael; Ray, Will; Sunyecz, Ian; Ueyama, Yukie
- Licensing Officer: Corris, Andrew
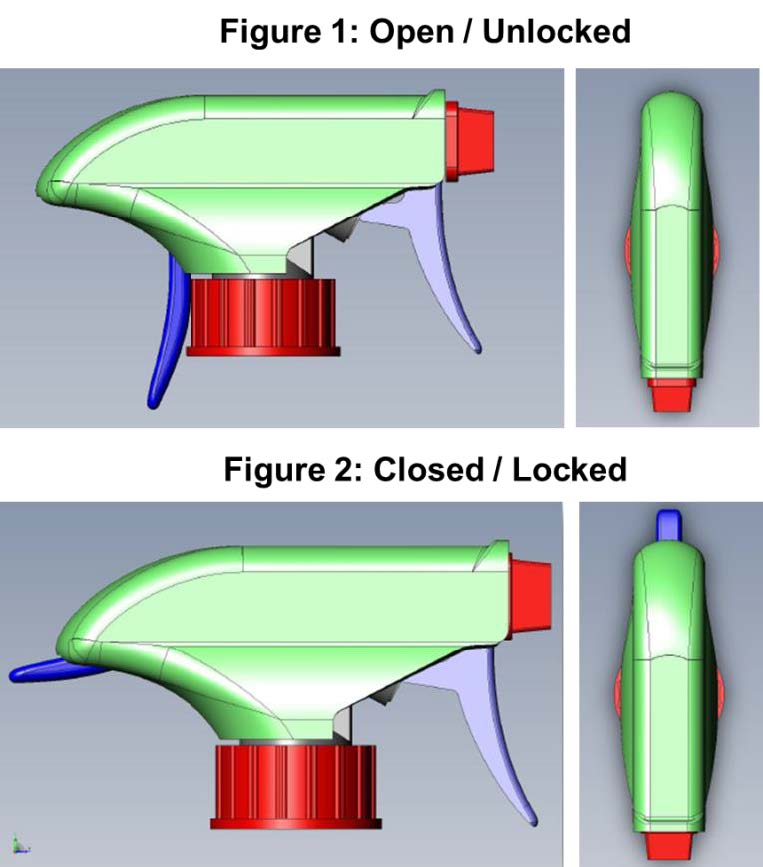 Child-Restraint Spray-Bottle for Household Cleaning Products
Child-Restraint Spray-Bottle for Household Cleaning Products
TS-001037 — When it comes to the safety of our children, innovation never stops. There have been many improvements to the safety of devices and receptacles that can be toxic or life threatening if consumed or exposed to skin. A team of researchers at Nationwide Children’s Hospital have incorporated this desire for security into a common household product: the spray bottle. Often filled with harmful chemicals, spray bottles remain one of the leading causes of chemical exposure injuries in children. The team at NCH has developed a “two-step authentication” spray nozzle that requires the dexterity beyond that of a small child. This dual trigger approach requires a full grip that prevents kids from accessing the contents of the spray bottle, while remaining easily usable by adults and seniors.
Benefits and Applications:
Inventors anticipate that incorporating this product into households will result in a decline in child injury due to accidental activation of spray bottles.
Stage of Development:
- College:
- Inventors: McKenzie, Lara; Nelson, Nicolas; Roberts, Kristin
- Licensing Officer: Murrah, Kyle
 A Virtual Reality Simulation to Aid in Exposure to Therapy for School Avoidance
A Virtual Reality Simulation to Aid in Exposure to Therapy for School Avoidance
TS-001036 — School can be a daunting experience. Constant motion, public speaking and a new environment can increase anxiety in children, sometimes leading to school avoidance. A team at Nationwide Children’s Hospital has developed a solution, where exposure therapy procedures are combined with modern technology can improve the school experience for people of all ages. Using a Virtual Reality (VR) Simulation, clinicians are able to use the multi-user capability to interact with and guide their patient through new environments such as classrooms, hallways and lunchrooms, as well as scenarios known to trigger increased anxiety such as public speaking or asking for help. Biofeedback components help collect data so that the clinician can adapt the experience to the user. Although targeted for school-aged children, this technology can be modified to treat any person with school or public phobia.
- College:
- Inventors: Huang, Yungui; DeForte, Shelly; Luna, John "John"; Mackner, Laura ; Vickery, Elizabeth
- Licensing Officer: Murrah, Kyle
 RyaBhata: A Shiny R Application for Single Cell Transcriptome Data Analysis and Visualization
RyaBhata: A Shiny R Application for Single Cell Transcriptome Data Analysis and Visualization
TS-001035 — Shiny R is an open source platform that allows a framework to develop online applications. With minimal required background in coding principles, a team of researchers at Nationwide Children’s Hospital were able to display and interact with the analysis made of single-cell transcriptome. Shiny R generates visualizations that include UMAP plots and presents features of single-cell RNA and transcriptomic data without extensive training in R programming. Improvements made on this existing technology includes importing data, cell filtration, principle component analysis, clustering, dimensional reduction, merging datasets, and Graphical user interface (GUI)-based generations of gene expression plots. This significantly improves the visualization and analysis of single-cell transcriptome analysis.
- College:
- Inventors: Manivannan, Sathiyanarayanan ; Garg, Vidu
- Licensing Officer: Murrah, Kyle
 Small Molecules that Inhibit and Disperse Salmonella Biofilms in vitro and are Active in Combination with Ciprofloxacin in vivo
Small Molecules that Inhibit and Disperse Salmonella Biofilms in vitro and are Active in Combination with Ciprofloxacin in vivo
TS-001034 — Salmonella is often caused by contact with animals that carry bacteria, contaminated food, or water. It has been observed that children are commonly afflicted by salmonella, and typically treatment includes fluids, medical care, and sometimes pharmaceuticals. A team of researchers at Nationwide Children’s hospital have identified a lead compound that includes a biofilm with anti-salmonella characteristics and acts as an inhibitor. Use of this compound with the antibiotic ciprofloxacin improves the elimination of bacterial infection in at-risk organs such as the liver and spleen.
- College:
- Inventors: Gunn, John; Sandala, Jenna
- Licensing Officer: Murrah, Kyle
 Automated Processing of Venous Intravascular Ultrasound Image
Automated Processing of Venous Intravascular Ultrasound Image
TS-001033 — Intravascular Ultrasound Images (IVUS) is a process that uses micro technology to provide images of blood vessels, their inner walls (endothelium) and the inside of veins. The analysis of these images allows clinicians to analyze luminal and scaffold boundaries, identify the presence of stenosis, and perform computations of various geometric quantities. This process is fully automated and therefore eliminates inconsistencies and inefficiencies that are a direct result of current semi-automated or complex fully automated systems already in place.
- College:
- Inventors: Ulziibayar, Anudari
- Licensing Officer: Murrah, Kyle
 Use of Tamoxifen to Reduce Breast Implant Capsule Formation and Capsular Contracture
Use of Tamoxifen to Reduce Breast Implant Capsule Formation and Capsular Contracture
TS-001029 — A major complication associated with breast implant prostheses is the occurrence of capsular contracture, occurring in 20%-25% of patients. Severe forms of capsular contracture constitute failure of the reconstruction with significant implications for increased cost owing to an increased need for recurrent medical interventions, as well diminished quality of life for patients. Capsular contracture occurs as the result of the patient’s immunologic ‘foreign body’ response to the implant material. The inventors’ vision is to develop a technology whereby the active metabolites of Tamoxifen (endoxifen) are conjugated to implant biomaterial in a manner allowing for localized delivery of endoxifen. They anticipate that local delivery of endoxifen will successfully reduce capsule formation around implant material by reducing the immunologic foreign body response. This is a technology that could be licensed to implant manufacturers (breast, implantable cardiac devices, etc.)
Technology Overview:
Breast augmentation and reconstruction is a common practice, especially in those afflicted by breast cancer. One of the most common issues that comes with this process is the formation of capsular contracture. This is a direct result of the patient’s immunologic ‘foreign body’ response to the implant material, which can impact the need for significant medical interventions and diminished quality of life. The current pharmacologic treatment for breast cancer is the chemical compound known as Tamoxifen, which acts as a chemo-preventative medication for hormone sensitive breast cancers. A team of researchers at Nationwide Children’s Hospital and Ohio State University aims to localize the delivery of tamoxifen to significantly reduce the immunologic foreign body response around implant material for use in both cancer-based breast reconstruction and cosmetic procedures
Benefits:
No pharmacotherapeutics currently exist to address capsular contracture and no biomaterial advances have been made to specifically reduce the foreign body response to breast implants
Stage of Development:
Mouse studies are currently underway using systemic delivery of Tamoxifen for treatment of capsular contracture in breast implants. A manuscript is in preparation.
Future mouse studies will focus on local delivery of endoxifen for treatment of capsular contracture in breast implants; then look at other implant types and different coating types.
Potential Applications / Markets:
According to the report published by Allied Market Research, the medical implant industry estimated $85.38 billion in 2019, and is estimated to generate $147.46 billion by 2027, manifesting a CAGR of 7.2% from 2020 to 2027. According to a report published by Fortune Business Insights, the breast implant market was worth $2.76 billion in 2019 and is projected to reach $3.05 billion by the end of 2027, exhibiting a CAGR of 7.2% during the forecast period, 2020-2027.
Opportunity / Seeking:
-Licensing
IP Status:
Patent application submitted
- College:
- Inventors: Blum, Kevin
- Licensing Officer: Murrah, Kyle
 Delivery of Adenosine Deaminase to Cancer Cells, Immune Cells and the Tumor Microenvironment
Delivery of Adenosine Deaminase to Cancer Cells, Immune Cells and the Tumor Microenvironment
TS-000973 — A recombinant oncolytic virus encoding either an adenosine deaminase or heterologous proteins can be used in treatment of a variety of diseases, as the primary purpose of these are to maintain and develop the immune system. A team of researchers at Nationwide Children’s Hospital have found a method that can address the delivery of adenosine deaminase into cancer cells, immune cells and the tumor microenvironment to aid in treatment for any disease or condition associated with adenosine or other associated markers.
- College:
- Inventors: Wang, Ruoning
- Licensing Officer: Zalucha, Ellen
 Overcoming Immune Checkpoint Inhibition with VISTA Deficient NK Cells – ViDe* NK Cells
Overcoming Immune Checkpoint Inhibition with VISTA Deficient NK Cells – ViDe* NK Cells
TS-000972 — Natural Killer (NK) cells express a range of receptors to activate or inhibit certain cellular behavior to kill cancer cells. When an NK cell is deficient or dysfunctional, the efficiency of the NK cells is severely limited. VISTA is a protein sequence that activates T cells and acts as a moderator for the immune system. It has low-to-moderate expression but has been the target of study by a team of researchers at Nationwide Children’s Hospital led by Dr. Dean Lee. By removing VISTA in expanded NK cells, the inhibitory signal will be eliminated and thus resulting in an enhanced ability of NK cells to target cancers and overcome the immune-suppressive signals for improved cancer immunotherapy.
- College:
- Inventors: Lee, Dean; Pereira, Marcelo
- Licensing Officer: Corris, Andrew
 Live Attenuated Mumps Virus-Based SARS-CoV-2 Vaccines for Infants and Children
Live Attenuated Mumps Virus-Based SARS-CoV-2 Vaccines for Infants and Children
TS-000971 — The novel disease Coronavirus, also denoted as COVID-19, was recognized by the World Health Organization as an unknown etiology in December of 2019. Severe Acute Respiratory Syndrome (SARS) is a disease that presents flu-like symptoms that is caused by coronavirus (SARS-CoV). The current pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is causing tremendous economical, emotional, and public health burdens. A team of researchers at Nationwide Children’s Hospital have reengineered a live attenuated recombinant mumps virus to create a novel SARS-CoV-2 vaccine for infants and children under the age of twelve. As vaccination is the most effective strategy to prevent infectious diseases, this development is instrumental to the outcome of the COVID-19 pandemic.
- College:
- Inventors: Peeples, Mark; KC, Mahesh
- Licensing Officer: Zalucha, Ellen
 Generation of Antigen-Specific Chimeric Antigen Receptor T cells Using Cas9/RNP and AAV
Generation of Antigen-Specific Chimeric Antigen Receptor T cells Using Cas9/RNP and AAV
TS-000969 — Gene therapy experts at Nationwide Children’s Hospital have made significant advancements in designing optimal viral vectors for producing Good Manufacturing Practice (GMP)-grade viral vector products. Adeno-associated virus (AAV) serotypes such as AAV1, 2, 2.5, 3, 5, 6, 8, 9 and rh74 have properties optimized by these researchers. Chimeric Antigen Receptor T cells (CAR T) are comprised of an extracellular antigen recognition domain, intracellular T cell activation and co-stimulatory domains. These cells allow for potent and specific targeting of cancer cells, bypassing the need for antigen presentation and T cell receptor recognition. Generating CAR T cells using the process of lentiviral transduction has limitations stemming from the random integration of transgenesis, where oncogene activation, gene silencing, or negative effects on the CAR T antitumor efficacy are possible.
- College:
- Inventors: Lee, Dean; Naeimi Kararoudi, Meisam
- Licensing Officer: Corris, Andrew
 Priming Peptide Pools for Isolation of SARS-CoV-2-Specific T Cells
Priming Peptide Pools for Isolation of SARS-CoV-2-Specific T Cells
TS-000913 — Peptides can be used to stimulate antigen-specific T cells, allowing activated T cells to be isolated from immune individuals to be used in vaccination or treatment in others. The novel disease Coronavirus, also denoted as 2019-nCoV, was recognized by the World Health Organization as an unknown etiology in December of 2019. Severe Acute Respiratory Syndrome (SARS) is a disease that presents flu-like symptoms that is caused by coronavirus (SARS-CoV). A current process widely applicable to many pathogens uses the Miltenyi Prodigy device. In a study led by Dr. Dean Lee, his team found that this process can be adapted to SARS-CoV-2 using a specialty mix of peptides to isolate T cell immunity.
- College:
- Inventors: Lee, Dean
- Licensing Officer: Corris, Andrew
 A Novel Compound, GQ-16 Protects Against Kidney Disease with Additional Insulin Sensitizing Benefits and Reduced Side Effects
A Novel Compound, GQ-16 Protects Against Kidney Disease with Additional Insulin Sensitizing Benefits and Reduced Side Effects
TS-000912 — There are a few prominent diseases that affect the kidney, such as nephrotic syndrome and diabetic nephropathy. To treat Type II diabetes, there is a readily available pharmaceutical known as pioglitazone that is often used in conjuncture with other compounds to reduce proteinuria in patients with kidney diseases. A team of researchers at Nationwide Children’s Hospital have developed a novel compound to act as a treatment agent in cases of kidney disease. The new design has similar insulin sensitizing effects as pioglitazone as well as its ability to reduce proteinuria. This compound, titled GQ-16, has similar efficacy as traditional Type II diabetes drugs and acts as a new indication for nephrotic syndrome or kidney diseases, with a significant reduction in side effects such as weight gain or adipogenesis.
- College:
- Inventors: Agrawal, Shipra
- Licensing Officer: Corris, Andrew
