Available Technologies
# of Displayed Technologies: 8 / 8
Applied Category Filter (Click To Remove): End User Innovation
Categories
 Sweat Technology for Monitoring Cystic Fibrosis Health and Adherence
Sweat Technology for Monitoring Cystic Fibrosis Health and Adherence
TS-001225 — Cystic fibrosis is an inherited disorder that affects cells that produce sweat and mucus, causing significant damage to the digestive system, lungs, and other organs. A team at Nationwide Children's Hospital has developed a non-invasive monitoring system to track and test a patient with this disease. This technology is a skin patch that measures the metabolomics of the patients sweat to evaluate the clinical health of patients afflicted with cystic fibrosis.
- College:
- Inventors: Hayes, Don; Kopp, Benjamin; Woodley, Frederick
- Licensing Officer: Murrah, Kyle
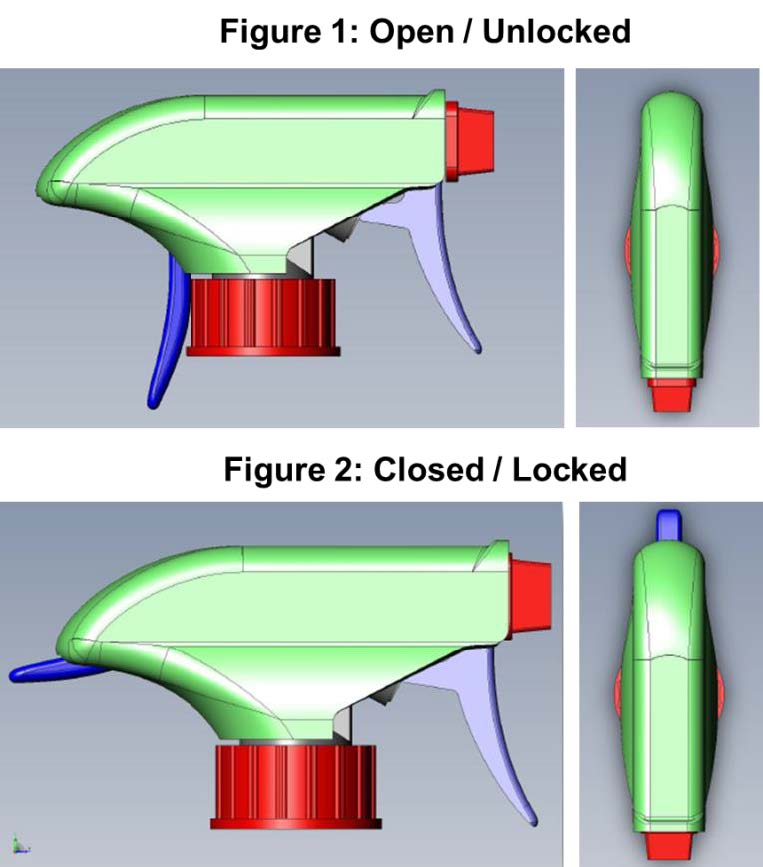 Child-Restraint Spray-Bottle for Household Cleaning Products
Child-Restraint Spray-Bottle for Household Cleaning Products
TS-001037 — When it comes to the safety of our children, innovation never stops. There have been many improvements to the safety of devices and receptacles that can be toxic or life threatening if consumed or exposed to skin. A team of researchers at Nationwide Children’s Hospital have incorporated this desire for security into a common household product: the spray bottle. Often filled with harmful chemicals, spray bottles remain one of the leading causes of chemical exposure injuries in children. The team at NCH has developed a “two-step authentication” spray nozzle that requires the dexterity beyond that of a small child. This dual trigger approach requires a full grip that prevents kids from accessing the contents of the spray bottle, while remaining easily usable by adults and seniors.
Benefits and Applications:
Inventors anticipate that incorporating this product into households will result in a decline in child injury due to accidental activation of spray bottles.
Stage of Development:
- College:
- Inventors: McKenzie, Lara; Nelson, Nicolas; Roberts, Kristin
- Licensing Officer: Murrah, Kyle
 A Virtual Reality Simulation to Aid in Exposure to Therapy for School Avoidance
A Virtual Reality Simulation to Aid in Exposure to Therapy for School Avoidance
TS-001036 — School can be a daunting experience. Constant motion, public speaking and a new environment can increase anxiety in children, sometimes leading to school avoidance. A team at Nationwide Children’s Hospital has developed a solution, where exposure therapy procedures are combined with modern technology can improve the school experience for people of all ages. Using a Virtual Reality (VR) Simulation, clinicians are able to use the multi-user capability to interact with and guide their patient through new environments such as classrooms, hallways and lunchrooms, as well as scenarios known to trigger increased anxiety such as public speaking or asking for help. Biofeedback components help collect data so that the clinician can adapt the experience to the user. Although targeted for school-aged children, this technology can be modified to treat any person with school or public phobia.
- College:
- Inventors: Huang, Yungui; DeForte, Shelly; Luna, John "John"; Mackner, Laura ; Vickery, Elizabeth
- Licensing Officer: Murrah, Kyle
.png) Virtual Reality-Based Pediatric Traumatic Brain Injury Assessment and Rehabilitation Platforms
Virtual Reality-Based Pediatric Traumatic Brain Injury Assessment and Rehabilitation Platforms
TS-000621 — Traumatic brain injury (TBI) is a leading cause of acquired disability in U.S. children and adolescents. Impairment of executive functions post-TBI has broad and profound implications for everyday life of pediatric patients, and the development of effective rehabilitation strategies is of significant clinical importance. Researchers at Nationwide Children’s Hospital have developed virtual reality (VR)-based programs for assessing cognitive function and providing subsequent rehabilitation. This pediatric TBI assessment software provides VR-based cognitive-assessment tasks and an additional training platform that pairs with the Oculus Rift virtual reality viewer. The training program is designed with a series of environmentally-enriched three-dimensional cognitive exercises that aid in rehabilitation of executive core functions among pediatric patients with TBI in a highly controlled, safe, and automated manner.
- College:
- Inventors: Xiang, Henry; Patterson, Jeremy; Shen, Jiabin
- Licensing Officer: Murrah, Kyle
 Transcranial Doppler Ultrasound Determination of Pathologic Mechanisms and Treatment Strategies for Cerebral Malaria
Transcranial Doppler Ultrasound Determination of Pathologic Mechanisms and Treatment Strategies for Cerebral Malaria
TS-000338 — Worldwide, malaria affects 2 million individuals annually. Cerebral malaria is the most severe neurological manifestation of malaria with case fatality rates ranging from 15-40%. Researchers at Nationwide Children’s Hospital have developed a method for using Transcranial Doppler to detect distinct waveform morphologies and identify pathogenic mechanisms leading to neuronal injury in children with cerebral malaria.
Benefits:
By identifying the specific sub-type of cerebral malaria, clinicians will be able to treat patients for their particular pathology, improving therapeutic outcomes.
Stage of Development
Following proof of concept studies, the researchers evaluated its use clinically in over 180 children with cerebral malaria and 140 control patients. The published study validated the use of transcranial Doppler ultrasound (TCD) in identifying distinct patient phenotypes which can serve as a guide for appropriate therapeutic regimens to specifically target the pathologic sub-type of cerebral malaria.
Potential Applications/Markets:
TCD can be used to identify pathogenic mechanisms leading to neuronal injury in children with cerebral malaria. We found 5 distinct waveform morphologies that are related to 5 different pathogenic mechanisms in children with malaria. To date, all clinical trials aimed at improving neurologic outcomes of these children have failed. We believe they have failed because drugs are not targeting the correct pathogenic mechanism in each child. In the future, TCD will be able to be used to determine which pathologic category individual children fall in and determine appropriate treatment strategies for them (clinical use). Additionally, this information is imperative to all future therapeutic trials looking at adjunctive agents aimed at the reduction of neurologic injury in these children research tool, probably before the clinical use).
Opportunity/Seeking:
Commercial Partner
Licensing
- College:
- Inventors: O'Brien, Nicole
- Licensing Officer: Murrah, Kyle
 Mobile Safety App
Mobile Safety App
TS-000161 — Customizable mobile app to reduce injury
Injuries are a major source of childhood emergency department and hospital admissions. Nearly 9000 children and young adults, aged 0-19 years, die from unintentional injuries each year. Known effective safety devices are readily available, however, there is no centralized and authoritative informati…
- College:
- Inventors: McKenzie, Lara; Roberts, Kristin
- Licensing Officer: Murrah, Kyle
 PS Rocker: A Multi-Head Skin Allergy Testing Device
PS Rocker: A Multi-Head Skin Allergy Testing Device
TS-000154 — There are 10 skin testing devices marketed in the United States for diagnosing allergies. These include single-tipped devices for testing allergens one at a time, as well as multi-head devices containing multiple testing tips on one device. One of the recently introduced multi-head devices is designed to decrease pain associated with skin testing. Current multi-head testing devices with fixed horizontal surfaces do not provide consistent intra-device contact with skin, while the single-prick devices can be impractical for children and time consuming. Clinicians at Nationwide Children’s Hospital and The Ohio State University, in collaborations with other independent inventors, have developed a new allergy skin testing device, PS Rocker, which improves upon existing products by combining the precision of a single prick test with the ease and speed of a multi-head device. Additionally, PS Rocker is less painful than traditional skin prick testing. The PS Rocker’s crescent-shaped, ergonomic design enables more reproducible tip contact with the skin than conventional horizontal multi-head devices, efficiently leading to more reliable results. Clinical studies of PS Rocker are currently underway at Nationwide Children’s Hospital testing effectiveness of PS Rocker.
- College:
- Inventors: Patterson, Amber; Patterson, Benjamin; Shepherd, Jay
- Licensing Officer: Murrah, Kyle
.png) Medical Line Safety Enclosure
Medical Line Safety Enclosure
TS-000104 — In health care settings, accidental suffocation and strangulation can occur due to medical line entanglement. Nurses at Nationwide Children’s Hospital have developed and clinically tested a novel medical line organizer that prevents accidental entanglement, suffocation, and strangulation of hospitalized individuals.
The medical line safety enclosure is a medical tubing or line organizer that prevents accidental entanglement, suffocation, or strangulation to hospital and other types of health care patients. The subject technology was originally developed and invented by a pediatric nurse, working within a clinical setting. According to February 2009's issue of Pediatrics, between 1984 and 2004, the infant mortality rates attributed to accidental suffocation and strangulation in beds increased from 2.8 to 12.5 deaths per 100,000 live births. In most cases of accidental strangulation, the tubing becomes entangled as the patient sleeps and rolls around in the crib or bed. Once the tubing is around the neck, it begins to close off the air supply of the patient, resulting in asphyxiation and possible death.
Similar asphyxiation cases are possible in other types of medical lines or tubing. For example ECG leads, pulse oximeter cables, and auto BP tubing can also become entangled around the patient. Several preventive measures, such as cable coiling or utilizing shorter tubing have been used with limited success.
Potential Fields of Use
The most direct application is in the prevention of injury or death due to strangulation and asphyxiation of pediatric patients being tangled or wrapped in their IV tubing or medical line. Other applications include use with geriatric patients as well in adult operating or surgical rooms and intensive care units.
Benefit Analysis
•Removes entanglement and asphyxiation risk in pediatric patients from IV tubing and other types of medical lines.
•Organizes medical tubing and lines into one single "bundle" for easy management and care.
•Proactively addresses safety and other liability issues with multi-line infusion therapy. No sharps, small parts, or metal in construction of device.
•Easy and less time consuming than other technologies in set-up and tear down for each use.
Stage of Development
The medical line safety enclosure has been field tested for several months within a clinical setting. It has a simple design and operation, requiring minimal development and investment. Further developments in material selection, fastening design and infection control parameters may be needed for final commercialization.
IP Status
A utility patent has been issued for this technology.
- College:
- Inventors: Mullet, Joyce; Ryan-Wenger, Nancy; Skeens, Micah
- Licensing Officer: Murrah, Kyle
