Available Technologies
# of Displayed Technologies: 15 / 15
Applied Category Filter (Click To Remove): End User Innovation
Categories
 Sweat Technology for Monitoring Cystic Fibrosis Health and Adherence
Sweat Technology for Monitoring Cystic Fibrosis Health and Adherence
TS-001225 — Cystic fibrosis is an inherited disorder that affects cells that produce sweat and mucus, causing significant damage to the digestive system, lungs, and other organs. A team at Nationwide Children's Hospital has developed a non-invasive monitoring system to track and test a patient with this disease. This technology is a skin patch that measures the metabolomics of the patients sweat to evaluate the clinical health of patients afflicted with cystic fibrosis.
- College:
- Inventors: Hayes, Don; Kopp, Benjamin; Woodley, Frederick
- Licensing Officer: Murrah, Kyle
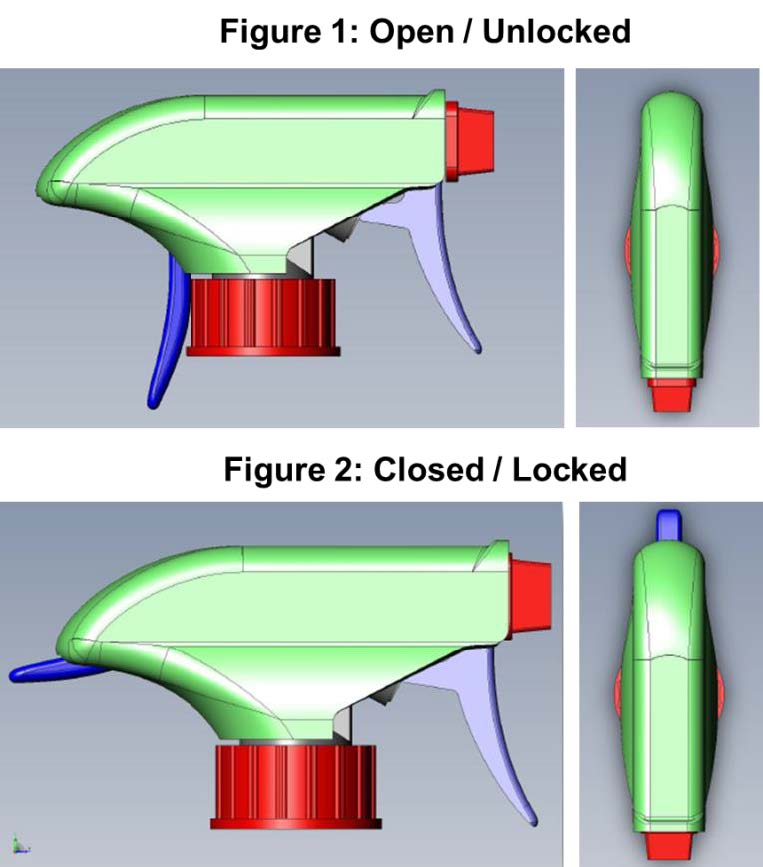 Child-Restraint Spray-Bottle for Household Cleaning Products
Child-Restraint Spray-Bottle for Household Cleaning Products
TS-001037 — When it comes to the safety of our children, innovation never stops. There have been many improvements to the safety of devices and receptacles that can be toxic or life threatening if consumed or exposed to skin. A team of researchers at Nationwide Children’s Hospital have incorporated this desire for security into a common household product: the spray bottle. Often filled with harmful chemicals, spray bottles remain one of the leading causes of chemical exposure injuries in children. The team at NCH has developed a “two-step authentication” spray nozzle that requires the dexterity beyond that of a small child. This dual trigger approach requires a full grip that prevents kids from accessing the contents of the spray bottle, while remaining easily usable by adults and seniors.
Benefits and Applications:
Inventors anticipate that incorporating this product into households will result in a decline in child injury due to accidental activation of spray bottles.
Stage of Development:
- College:
- Inventors: McKenzie, Lara; Nelson, Nicolas; Roberts, Kristin
- Licensing Officer: Murrah, Kyle
 A Virtual Reality Simulation to Aid in Exposure to Therapy for School Avoidance
A Virtual Reality Simulation to Aid in Exposure to Therapy for School Avoidance
TS-001036 — School can be a daunting experience. Constant motion, public speaking and a new environment can increase anxiety in children, sometimes leading to school avoidance. A team at Nationwide Children’s Hospital has developed a solution, where exposure therapy procedures are combined with modern technology can improve the school experience for people of all ages. Using a Virtual Reality (VR) Simulation, clinicians are able to use the multi-user capability to interact with and guide their patient through new environments such as classrooms, hallways and lunchrooms, as well as scenarios known to trigger increased anxiety such as public speaking or asking for help. Biofeedback components help collect data so that the clinician can adapt the experience to the user. Although targeted for school-aged children, this technology can be modified to treat any person with school or public phobia.
- College:
- Inventors: Huang, Yungui; DeForte, Shelly; Luna, John "John"; Mackner, Laura ; Vickery, Elizabeth
- Licensing Officer: Murrah, Kyle
.png) Virtual Reality-Based Pediatric Traumatic Brain Injury Assessment and Rehabilitation Platforms
Virtual Reality-Based Pediatric Traumatic Brain Injury Assessment and Rehabilitation Platforms
TS-000621 — Traumatic brain injury (TBI) is a leading cause of acquired disability in U.S. children and adolescents. Impairment of executive functions post-TBI has broad and profound implications for everyday life of pediatric patients, and the development of effective rehabilitation strategies is of significant clinical importance. Researchers at Nationwide Children’s Hospital have developed virtual reality (VR)-based programs for assessing cognitive function and providing subsequent rehabilitation. This pediatric TBI assessment software provides VR-based cognitive-assessment tasks and an additional training platform that pairs with the Oculus Rift virtual reality viewer. The training program is designed with a series of environmentally-enriched three-dimensional cognitive exercises that aid in rehabilitation of executive core functions among pediatric patients with TBI in a highly controlled, safe, and automated manner.
- College:
- Inventors: Xiang, Henry; Patterson, Jeremy; Shen, Jiabin
- Licensing Officer: Murrah, Kyle
 Stuttering Treatment Monitoring App
Stuttering Treatment Monitoring App
TS-000540 — A team led by Dr. Christopher Bartlett at Nationwide Children’s Hospital has developed an app to connect patients with a personalized plan created by speech therapists to aid those with a stutter. This app combines audio-visual components with text to explain an exercise, which is then recorded as the client performs it. The team expanded on this concept to include a deep neural network to provide immediate feedback, as well as the option to connect and share results on social media and with their therapist. The care provider will be able to add exercises as their patient progresses. Once an exercise is mastered, the exercise is opened permanently to the client.
- College:
- Inventors: Bartlett, Christopher; Bambach, Sven; Huang, Yungui; Oiler, Brannon
- Licensing Officer: Corris, Andrew
 All in One IV pole: Tape dispenser, sharps bins and medical trash containers
All in One IV pole: Tape dispenser, sharps bins and medical trash containers
TS-000435 — Inventors at Nationwide Children’s Hospital have developed a device consisting of an intravenous pole (IV) that harbors a clamp for adhesive tape dispenser, sharp bins and medical trash container. This prototype “all in one” equipment is a convenient and practical site for adhesive tape dispensation and surgical trash disposal while ensuring patient safety in the operating suite.
- College:
- Inventors: Hudson, Rick; Kelley, Adam
- Licensing Officer: Eidahl, Jocelyn
.png) "Facing Takeoff" - A Day-Long Workshop for Those Interested in Easing their Fear of Flying
"Facing Takeoff" - A Day-Long Workshop for Those Interested in Easing their Fear of Flying
TS-000433 — Flying phobia is a highly prevalent anxiety disorder, which causes sufferers significant distress and life interference. There are multiple interventions addressing flying anxiety provided through airports, commercial airlines as well as online materials. However, these services either charge fees or do not provide any aircraft/airport simulation experience. Inventors at Nationwide Children’s Hospital have designed a unique workshop curriculum which includes PowerPoint slides, a video tape and participant handouts and experimental activities. This educational intervention facilitates increasing tolerance of and comfort with air travel.
- College:
- Inventors: Bloomster, Brent
- Licensing Officer: Corris, Andrew
 Transcranial Doppler Ultrasound Determination of Pathologic Mechanisms and Treatment Strategies for Cerebral Malaria
Transcranial Doppler Ultrasound Determination of Pathologic Mechanisms and Treatment Strategies for Cerebral Malaria
TS-000338 — Worldwide, malaria affects 2 million individuals annually. Cerebral malaria is the most severe neurological manifestation of malaria with case fatality rates ranging from 15-40%. Researchers at Nationwide Children’s Hospital have developed a method for using Transcranial Doppler to detect distinct waveform morphologies and identify pathogenic mechanisms leading to neuronal injury in children with cerebral malaria.
Benefits:
By identifying the specific sub-type of cerebral malaria, clinicians will be able to treat patients for their particular pathology, improving therapeutic outcomes.
Stage of Development
Following proof of concept studies, the researchers evaluated its use clinically in over 180 children with cerebral malaria and 140 control patients. The published study validated the use of transcranial Doppler ultrasound (TCD) in identifying distinct patient phenotypes which can serve as a guide for appropriate therapeutic regimens to specifically target the pathologic sub-type of cerebral malaria.
Potential Applications/Markets:
TCD can be used to identify pathogenic mechanisms leading to neuronal injury in children with cerebral malaria. We found 5 distinct waveform morphologies that are related to 5 different pathogenic mechanisms in children with malaria. To date, all clinical trials aimed at improving neurologic outcomes of these children have failed. We believe they have failed because drugs are not targeting the correct pathogenic mechanism in each child. In the future, TCD will be able to be used to determine which pathologic category individual children fall in and determine appropriate treatment strategies for them (clinical use). Additionally, this information is imperative to all future therapeutic trials looking at adjunctive agents aimed at the reduction of neurologic injury in these children research tool, probably before the clinical use).
Opportunity/Seeking:
Commercial Partner
Licensing
- College:
- Inventors: O'Brien, Nicole
- Licensing Officer: Murrah, Kyle
.png) Computerized Screening Tool for Behavioral Health (Version 2)
Computerized Screening Tool for Behavioral Health (Version 2)
TS-000303 — Despite the high prevalence of mental health, many cases go without treatment- in part because their disorders go undiagnosed. Scientists at Nationwide Children’s Hospital have developed a computerized screening tool for behavioral health that is able to administer and score a set of mental health symptom questionnaires. This web application enables earlier identification of mental health disorders leading to earlier care.
This is an improvement to the software platform for completing behavioral health screenings (questionnaires that assess symptoms of and impairment due to mental health problems). The "second generation" system is currently built to work from the NCH Enterprise Data Warehouse.
- College:
- Inventors: Krishna, Rajeev
- Licensing Officer: Corris, Andrew
.png) pNLRep2-Cap5kan
pNLRep2-Cap5kan
TS-000289 — Gene therapy experts at Nationwide Children’s Hospital have made significant advancements in designing optimal viral vectors for producing Good Manufacturing Practice (GMP)-grade viral vector products. Our experts have optimized properties of vectors for a wide variety of Adeno-associated virus (AAV) serotypes, including AAV1, 2, 2.5, 3, 5, 6, 8, 9 and rh74. In particular, our experts have optimized virus packaging efficiency, reduced potential to form replication competent AAV and replaced the beta-lactam resistant gene with kanamycin in order to be compliant with European Union (EU) regulations. Our experts have made additional optimized vectors for AAVrh74, and AAV9 that allow for more efficient purification and improved CNS transduction, respectively.
- College:
- Inventors: Loiler, Scott
- Licensing Officer: Corris, Andrew
.png) Hear Me Read Application for Children with Hearing Loss
Hear Me Read Application for Children with Hearing Loss
TS-000278 — Background / Context / Abstract:
Nearly 34 million children globally have disabling hearing loss, facing challenges in developing spoken language and literacy. Expert therapy is needed to achieve success, and no digital therapy interventions designed for deaf/hard of hearing (D/HH) children are available.
Technology Overview:
We have created a novel mobile app “Hear Me Read” designed to use digital storybooks as audio and video tools in therapy, in a platform that enhances family engagement for D/HH children.
Therapists can select stories based on reading level, auditory and grammatical features, then assign them to parents with embedded individualized prompts and therapeutic goals. Parents can create personalized narrations of a book, explore vocabulary and the embedded therapist prompts and tasks.
Our unique team is made up of pediatric hearing loss professionals (speech and language therapists, ENT surgeon), a parent of a D/HH child, and a software designer, all of whom incorporated design elements tailored to the specific learning needs of these children. Learn how “Hear Me Read” was developed to help at-risk children use storybooks in a single app to achieve multiple therapy goals over time!
Benefits:
The Hear Me Read platform is a modern and adaptable reading intervention tailored to the needs of D/HH children. We expect that HMR can improve spoken language and literacy outcomes in young children who are D/HH and greatly increase access to evidence-based and expert-driven therapy in those who could not otherwise attain it. We also expect to use HMR as a research tool to gain insights in literacy, accelerate and lead development of novel future reading interventions, thereby transforming therapy for D/HH (and other at risk) children.
Further Details / State of Development:
We have a currently functional iOS based app on an iPad platform. We have executed surveys and focus groups in speech-language pathologists and parents of D/HH children to confirm value of the product, and provide feedback on the technology (which we have incorporated).
We are currently investigating the efficacy of Hear Me Read in a prospective clinical trial, which provides a unique evidence-based foundation for this technology.
We are ready to move from a Minimal Viable Product to an active development phase to upgrade our software strategy, cloud-based access, and delivery model.
Potential Applications / Potential Markets:
We anticipate this technology to apply more broadly to children beyond D/HH, but to include other at-risk children. This may include children with:
Autism Spectrum Disorder
Dyslexia
Specific Reading Disorder
Other Reading Disorders
End users may include parents of these children, as well as speech-language therapists. Teachers and schools may be secondary markets.
Opportunity / Seeking:
☒Development partner
☒Commercial partner
☒Licensing
☒University spin out
☒Seeking investment
We are exploring all options at this time.
IP Status:
☐Patented
☒Patent application submitted
☒Provisional patent
☐No patent
☐Know-how based
☒Copyright
- College:
- Inventors: Malhotra, Prashant; Huefner, Janelle; Lucius, Shana; Luna, John "John"; Satyapriya, Anand
- Licensing Officer: Corris, Andrew
 Mobile Safety App
Mobile Safety App
TS-000161 — Customizable mobile app to reduce injury
Injuries are a major source of childhood emergency department and hospital admissions. Nearly 9000 children and young adults, aged 0-19 years, die from unintentional injuries each year. Known effective safety devices are readily available, however, there is no centralized and authoritative informati…
- College:
- Inventors: McKenzie, Lara; Roberts, Kristin
- Licensing Officer: Murrah, Kyle
 PS Rocker: A Multi-Head Skin Allergy Testing Device
PS Rocker: A Multi-Head Skin Allergy Testing Device
TS-000154 — There are 10 skin testing devices marketed in the United States for diagnosing allergies. These include single-tipped devices for testing allergens one at a time, as well as multi-head devices containing multiple testing tips on one device. One of the recently introduced multi-head devices is designed to decrease pain associated with skin testing. Current multi-head testing devices with fixed horizontal surfaces do not provide consistent intra-device contact with skin, while the single-prick devices can be impractical for children and time consuming. Clinicians at Nationwide Children’s Hospital and The Ohio State University, in collaborations with other independent inventors, have developed a new allergy skin testing device, PS Rocker, which improves upon existing products by combining the precision of a single prick test with the ease and speed of a multi-head device. Additionally, PS Rocker is less painful than traditional skin prick testing. The PS Rocker’s crescent-shaped, ergonomic design enables more reproducible tip contact with the skin than conventional horizontal multi-head devices, efficiently leading to more reliable results. Clinical studies of PS Rocker are currently underway at Nationwide Children’s Hospital testing effectiveness of PS Rocker.
- College:
- Inventors: Patterson, Amber; Patterson, Benjamin; Shepherd, Jay
- Licensing Officer: Murrah, Kyle
.png) CHURCHILL: A Comprehensive Analysis Pipeline for the Discovery of Human Genetic Variation
CHURCHILL: A Comprehensive Analysis Pipeline for the Discovery of Human Genetic Variation
TS-000141 — Next Generation Sequencing (NGS) is a sequencing technology characterized by the large volume of generated DNA/RNA sequencing data. However, the cost to analyze NGS data is outstripping the cost to actually produce the data. To circumvent this challenge, the Institute for Genomics Medicine have developed a fully automated and comprehensive analysis tool called CHURCHILL. CHURCHILL’s patented novel parallelization strategy analyzes raw NGS data in a record time.
- College:
- Inventors: White, Peter; Fitch, James; Hu, Patrick (Yangqiu) ; Kelly, Ben; Newsom, David
- Licensing Officer: Corris, Andrew
.png) Medical Line Safety Enclosure
Medical Line Safety Enclosure
TS-000104 — In health care settings, accidental suffocation and strangulation can occur due to medical line entanglement. Nurses at Nationwide Children’s Hospital have developed and clinically tested a novel medical line organizer that prevents accidental entanglement, suffocation, and strangulation of hospitalized individuals.
The medical line safety enclosure is a medical tubing or line organizer that prevents accidental entanglement, suffocation, or strangulation to hospital and other types of health care patients. The subject technology was originally developed and invented by a pediatric nurse, working within a clinical setting. According to February 2009's issue of Pediatrics, between 1984 and 2004, the infant mortality rates attributed to accidental suffocation and strangulation in beds increased from 2.8 to 12.5 deaths per 100,000 live births. In most cases of accidental strangulation, the tubing becomes entangled as the patient sleeps and rolls around in the crib or bed. Once the tubing is around the neck, it begins to close off the air supply of the patient, resulting in asphyxiation and possible death.
Similar asphyxiation cases are possible in other types of medical lines or tubing. For example ECG leads, pulse oximeter cables, and auto BP tubing can also become entangled around the patient. Several preventive measures, such as cable coiling or utilizing shorter tubing have been used with limited success.
Potential Fields of Use
The most direct application is in the prevention of injury or death due to strangulation and asphyxiation of pediatric patients being tangled or wrapped in their IV tubing or medical line. Other applications include use with geriatric patients as well in adult operating or surgical rooms and intensive care units.
Benefit Analysis
•Removes entanglement and asphyxiation risk in pediatric patients from IV tubing and other types of medical lines.
•Organizes medical tubing and lines into one single "bundle" for easy management and care.
•Proactively addresses safety and other liability issues with multi-line infusion therapy. No sharps, small parts, or metal in construction of device.
•Easy and less time consuming than other technologies in set-up and tear down for each use.
Stage of Development
The medical line safety enclosure has been field tested for several months within a clinical setting. It has a simple design and operation, requiring minimal development and investment. Further developments in material selection, fastening design and infection control parameters may be needed for final commercialization.
IP Status
A utility patent has been issued for this technology.
- College:
- Inventors: Mullet, Joyce; Ryan-Wenger, Nancy; Skeens, Micah
- Licensing Officer: Murrah, Kyle
